Europe Nanomedicine Market Size, Share, Trends & Growth Forecast Report By Product, Disease Type, Nanomolecule Type and Country (UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic and Rest of Europe) - Industry Analysis, From (2025 to 2033)
Europe Nanomedicine Market Size
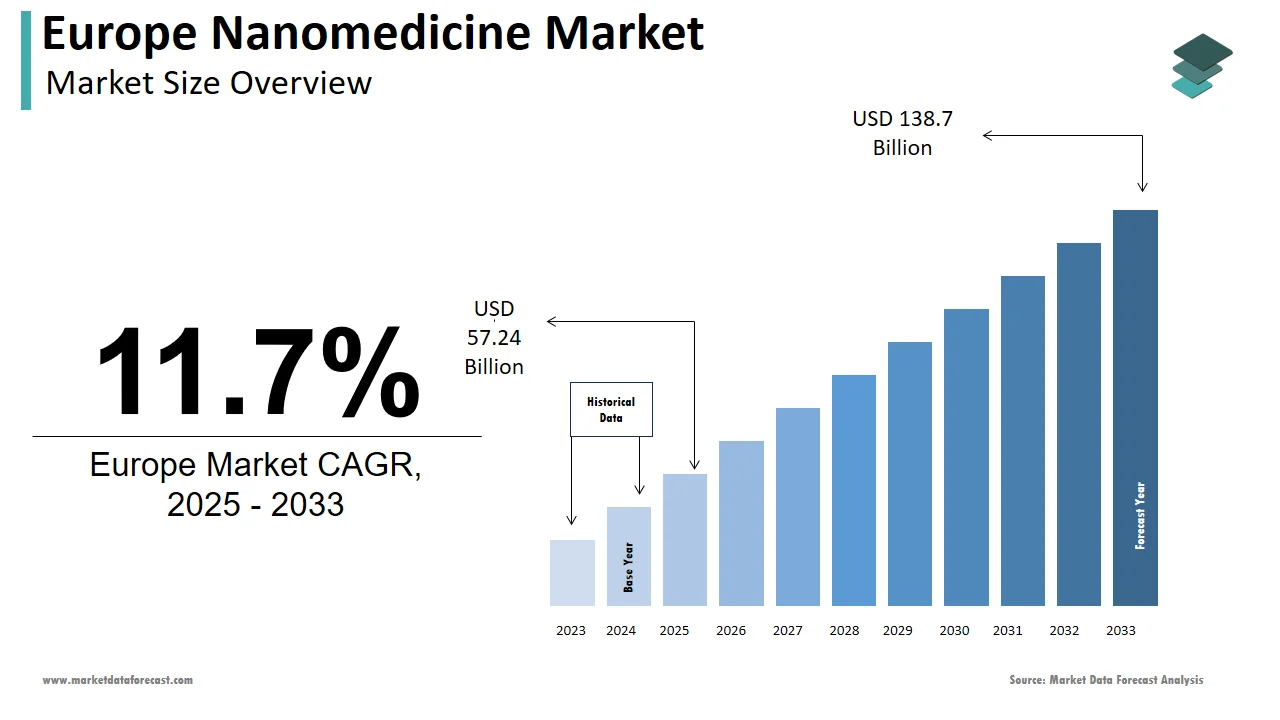
The nanomedicine market size in Europe was valued at USD 51.24 billion in 2024. The European market is estimated to be worth USD 138.7 billion by 2033 from USD 57.24 billion in 2025, growing at a CAGR of 11.7% from 2025 to 2033.
MARKET DRIVERS
Emerging Technologies in Drug Delivery
Emerging technologies for drug delivery, increased use of nanomedicine across various applications, increased government support and funding, increased demand for therapies with fewer side effects, and cost-effectiveness of therapies drive the European nanomedicine market. Cancer, Parkinson's disease, Alzheimer's disease, diabetes, orthopedic illnesses, and diseases of the blood, lungs, and cardiovascular system benefit from nanomedicine. Using nanotechnology-based contrast reagents for diagnosis and monitoring pharmacological effects on an unprecedentedly short timescale is also expected to fuel future growth. Demand is also expected to be influenced by the demand for biodegradable implants with an extended lifespan for wound healing. In addition, Nanomedicine improves medicine delivery by manipulating materials at the nanoscale. As a result, nanomedicine has aided in the treatment of a variety of diseases.
Increase in Nano-Drug Out-Licensing
The increase in nano-drug out-licensing and expanding healthcare facilities in this nation are expected to give several opportunities for the European nanomedicine industry to grow. Nano formulations with the triggered release for custom pharmacokinetics, nanoparticles for local tumor control in combination with radiotherapy, and functionalized nanoparticles for targeted in-vivo activation of stem cell production are expected to drive R&D in the coming year's revenue generation. Nanomedicine could change cancer therapy. This rapidly expanding field of medical study can lead to more personalized treatments for various diseases, including cancer. Furthermore, the market's expansion is fuelled by increased participants and the widespread adoption of innovative technologies. Furthermore, the country's robust government commitment to expanding healthcare facilities through insurance plans is expected to generate lucrative growth opportunities.
MARKET RESTRAINTS
Long Approval Process
The growth of the European nanomedicine market is limited by the long approval process and the hazards connected with nanomedicine. In addition, the market's expansion is being hampered by severe regulatory concerns and the high cost of nanoparticle-assisted medicine compared to its traditional counterparts.
Tedious Lab-to-Market Process
The entire process of the lab-to-market approval for nanotechnology applications is tedious and expensive, with severe regulatory evaluations involved, causing investors to be extremely cautious about investing. Nanomedicine is still in its infancy, with various items in development. However, challenges have hampered the market's full potential, including costly research and development (R&D), regulatory issues where detailed guidelines were not always supplied, and the nanoparticles' safety. It will be vital for this market to overcome these challenges if it continues to grow.
REGIONAL ANALYSIS
Geographically, Europe is home to several major companies operating in nanomedicine, resulting in high market penetration and awareness among people. The United Kingdom leads the nanomedicine market in Europe. As a result, Europe is expected to have favorable growth during the forecast period. The increased adoption of nanomedicine by healthcare professionals over traditional drugs, the improved and growing number of nanomedicines, and changing regulatory dynamics that allow more nanomedicine candidates to be reviewed for approval are all key drivers of this market's market continued growth. In addition, the growing prevalence of cancer, genetic and cardiovascular diseases, and increasing advancements in nanoscale technologies for diagnostic processes are all driving the growth of the European nanomedicine industry.
KEY MARKET PLAYERS
Johnson & Johnson, Merck & Co., Pfizer, Abbott Laboratories, GE Healthcare, Celgene Corporation, Nanosphere, Inc, Gilead Sciences, Hoffmann-La Roche, Nanospectra Biosciences, and Arrowhead Pharmaceuticals are some of the noteworthy companies in the European nanomedicine market.
MARKET SEGMENTATION
This Europe nanomedicine market research report is segmented and sub-segmented into the following categories.
By Product
- Diagnostic Imaging
- Regenerative Medicine
- Drug Delivery
- Implants
- Vaccines
- Others
By Disease Type
- Cardiovascular Diseases
- Neurological Diseases
- Ophthalmological Diseases
- Infectious Diseases
- Immunological Diseases
- Orthopedic Disorders
- Oncological Diseases
- Others
By Nanomolecule Type
- Nanotubes
- Nanoparticles
- Nanodevices
- Nanoshells
- Others
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Frequently Asked Questions
What was the size of the European nanomedicine market in 2024?
The European nanomedicine market size was worth USD 51.24 billion in 2024.
What factors are driving the growth of the Europe nanomedicine market?
The growing prevalence of chronic diseases, rising healthcare expenditure, technological advancements in nanomedicine, and the growing demand for personalized medicine are propelling the European nanomedicine market.
What are the major players in the Europe nanomedicine market?
Merck & Co., Inc., Hoffmann-La Roche Ltd., Abbott Laboratories, Novartis AG, and Pfizer, Inc. are playing a noteworthy role in the European nanomedicine market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]