Europe Cardiac Surgery Devices Market Size, Share, Trends & Growth Forecast Report By Product Type, Application, Age Group and Country (UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic and Rest of Europe) - Industry Analysis, From (2025 to 2033)
Europe Cardiac Surgery Devices Market Size
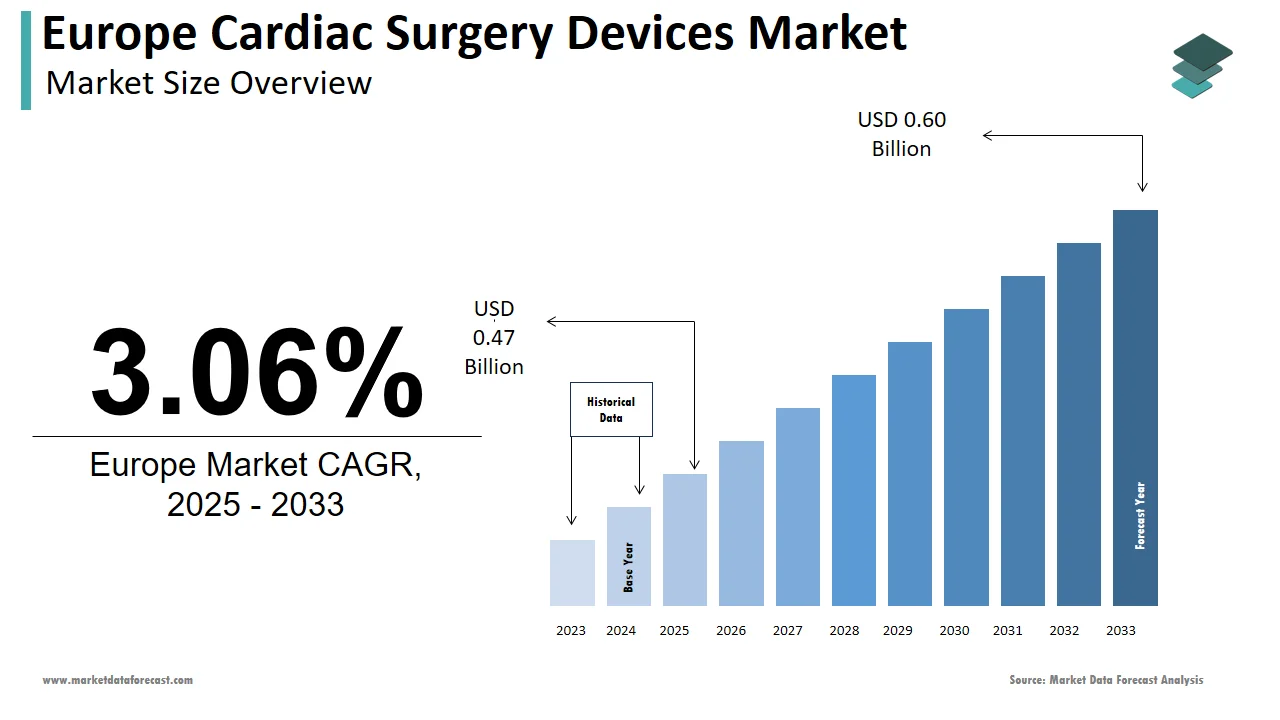
The cardiac surgery devices market size in Europe was valued at USD 0.46 billion in 2024. The European market is estimated to be worth USD 0.60 billion by 2033 from USD 0.47 billion in 2025, growing at a CAGR of 3.06% from 2025 to 2033.
MARKET DRIVERS
Rising Chronic Disease Burden
A rise in the occurrence rate of diabetes, atherosclerosis, stroke, hypertension, and other chronic diseases in people's habits and adopting sedentary changes are likely to boost the cardiac surgery devices market growth in the analysis period. Owing to increasing cardiovascular diseases and the launch of wireless ECG, surgeons can monitor and identify the patient remotely, accelerating the market growth in this region over the review period. Continuous research related to cardiac failure, heart attacks, and other heart relevant ailments is projected to surge the market. Steps taken by the government organizations to increase the awareness related to cardiac surgeries are fostering the market. FDA has accepted the third-version of Abbott Laboratories, MitraClip device, which is a minimally invasive alternative to open-heart surgery for patients with leakage of mitral valves.
MARKET RESTRAINTS
High Cost of Devices and Treatment
The cost related to the devices or the instruments at the time of heart relevant surgeries and the treatment process are so expensive that people can't afford are factors hampering the market growth. Strict rules and regulatory policies for the approval and acceptance of new devices into the market are to impede the growth in this region. The adoption of substitute surgical methods is to hinder the market.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Product Type, Application, Age Group, and Region. |
|
Various Analyses Covered |
Global, Regional and Country-Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Countries Covered |
UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, the Czech Republic, and the Rest of Europe. |
|
Market Leaders Profiled |
Trinity Biotech, Abiomed, Inc., Cardinal Health, C. R. Bard, Inc., MedWaves Incorporated, CyberHeart Incorporated, and Transmedics, Inc., among others. |
REGIONAL ANALYSIS
Europe was the third-largest market for cardiac surgery devices in the world by the share in 2024. Factors such as the growing prevalence of heart diseases and rising healthcare expenditure are expected to drive market growth during the forecast period.
Europe is anticipated to have a significant share in the cardiac surgery devices market during the analysis period. The European market is attributed to factors like the rise in the aging population. It is predicted to have growth in requirements for surgical procedures, enhance the healthcare domain, and increase the obtainment of developed medical products. Germany is considered to lead the cardiac surgery devices market with a CAGR of 5.6% market share over the period. As per the report submitted by Eurostat, nearly 48,0 00 heart bypass surgeries were implemented in Germany. This country has registered a considerable number of operations, increasing demand for minimally invasive surgeries, and advanced technologies are expected to stimulate this region's market. France's market is projected to have notable growth in cardiac surgery devices due to increasing awareness of different diseases and the growing number of older people in the region.
KEY MARKET PLAYERS
Key players operating in the Europe cardiac surgery devices market profiled in this report are Trinity Biotech, Abiomed, Inc., Cardinal Health, C. R. Bard, Inc., MedWaves Incorporated, CyberHeart Incorporated, and Transmedics, Inc., among others.
MARKET SEGMENTATION
This Europe cardiac surgery devices market research report is segmented and sub-segmented into the following categories.
By Product Type
- Cardiopulmonary Bypass Equipment
- Beating Heart Surgery Systems
- Perfusion Disposables
- and Cardiac Ablation Devices
By Application
- Cardiac Arrhythmia
- Coronary Heart Disease
- Congenital Heart Defects
- Congestive Heart Failure
- Other Applications
By Age Group
- New-born (0-30 Days)
- Infant (31 Days-1 Year)
- Children (1-18 Years)
- Adult (18+ Years)
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]