Europe Prenatal And Neonatal Equipment Care Market Research Report - Segmented By Product Type & By Country(UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic & Rest of Europe) - Industry Analysis (2025 to 2033)
Europe Prenatal and Neonatal Equipment Care Market Size
The Europe Prenatal And Neonatal Equipment Care Market Size was valued at USD 2.19 billion in 2024. The Europe Prenatal And Neonatal Equipment Care Market Size is expected to have 6.25% CAGR from 2025 to 2033 and be worth USD 3.78 billion by 2033 from USD 2.33 billion in 2025.
The prenatal and neonatal equipment care market encompasses a range of specialized medical devices and support systems designed to monitor, diagnose, and treat expectant mothers and newborn infants. These include fetal monitors, incubators, phototherapy units, neonatal ventilators, maternal diagnostic imaging systems, and various accessories essential for ensuring safe pregnancies and healthy births. In Europe, this sector plays a critical role in healthcare infrastructure, particularly as maternal and neonatal health outcomes are closely monitored by national health authorities and international organizations.
Europe’s healthcare system is characterized by high standards of maternal and infant care, supported by well-established public and private hospital networks.
The growing prevalence of high-risk pregnancies—linked to rising maternal age, obesity, and chronic diseases—has further increased reliance on advanced care equipment.
Moreover, technological innovation remains a key focus area. Institutions such as the European Foundation for the Care of Newborn Infants (EFCNI) and the UK’s National Institute for Health and Care Excellence (NICE) have advocated for greater investment in digital monitoring, remote diagnostics, and AI-assisted fetal assessments.
MARKET DRIVERS
Increasing Incidence of Preterm Births and Neonatal Complications
One of the primary drivers fueling the growth of the Europe prenatal and neonatal equipment care market is the rising incidence of preterm births and associated neonatal complications. According to the European Perinatal Health Report published by Euro-Peristat, preterm birth rates across EU countries ranged between 5% and 12% in 2023 , varying by country but showing an upward trend compared to previous decades.
Preterm infants often require extended stays in neonatal intensive care units (NICUs), where specialized equipment such as incubators, radiant warmers, ventilators, and phototherapy units are essential for survival and development. As per data from the World Health Organization (WHO), babies born before 37 weeks of gestation account for over 70% of neonatal deaths globally , underscoring the importance of timely and effective neonatal interventions.
In response, European hospitals have significantly upgraded their NICU facilities.
Also, research initiatives such as the EU-funded NeoGrowth project are focusing on improving neonatal outcomes through advanced nutritional support and respiratory management technologies. With the number of preterm births projected to rise due to changing demographics and lifestyle factors, the demand for sophisticated prenatal and neonatal equipment is expected to grow steadily across the region.
Advancements in Fetal Monitoring and Diagnostic Technologies
Another major driver influencing the Europe prenatal and neonatal equipment care market is the rapid advancement in fetal monitoring and diagnostic technologies. Innovations in ultrasound imaging, Doppler systems, cardiotocography (CTG), and non-invasive prenatal testing (NIPT) have significantly enhanced the ability to detect fetal abnormalities and manage high-risk pregnancies effectively.
Moreover, companies like Philips Healthcare, GE Healthcare, and Siemens Healthineers have introduced cloud-connected fetal monitoring platforms that enable remote access to maternal and fetal health data. As noted by the British Maternal and Fetal Medicine Society, these digital solutions have proven especially valuable in rural and semi-urban settings, where specialist obstetricians may not be immediately available .
The integration of portable and handheld Doppler devices into routine prenatal checkups has also expanded access to early detection of intrauterine growth restrictions and placental issues.
With continued investment in smart diagnostics and telemedicine-enabled prenatal care, the demand for advanced monitoring equipment is set to expand, reinforcing the growth trajectory of the European prenatal and neonatal equipment care market.
MARKET RESTRAINTS
High Cost of Advanced Neonatal and Prenatal Equipment
A major restraint affecting the expansion of the Europe prenatal and neonatal equipment care market is the high cost associated with acquiring and maintaining advanced medical devices. Despite Europe's strong healthcare infrastructure, budgetary constraints at both national and institutional levels limit widespread adoption, particularly in smaller hospitals and regional clinics.
Furthermore, maintenance, calibration, and software upgrades contribute to long-term operational costs.
While wealthier nations such as Germany, France, and the UK can absorb these costs through public funding and insurance mechanisms, lower-income EU countries face challenges in sustaining high-tech neonatal services. Until financing models become more flexible and cost-effective alternatives emerge, affordability will remain a key barrier to the broader deployment of advanced prenatal and neonatal care equipment across Europe.
Regulatory Complexity and Lengthy Approval Processes
Another significant constraint impacting the Europe prenatal and neonatal equipment care market is the regulatory complexity and lengthy approval processes required for medical device commercialization. The Medical Device Regulation (MDR) framework introduced by the European Union in 2021 mandates rigorous conformity assessments, clinical evaluations, and post-market surveillance, leading to extended timelines for product launches.
As per a 2024 analysis by the European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry (COCIR), the average time required to obtain CE marking for Class IIb and Class III neonatal and prenatal devices increased from 12 months to 18–24 months following the implementation of the MDR , delaying market entry for new products.
This regulatory landscape disproportionately affects small and mid-sized manufacturers who lack the resources to navigate complex compliance requirements.
Apart from these, variations in national implementation of EU-wide regulations create inconsistencies across markets. As per the French Ministry of Health, some regions in France experienced prolonged delays in adopting new fetal monitoring devices due to differing interpretations of regulatory guidelines at the hospital level .
While these regulations aim to ensure patient safety, they pose considerable barriers to market agility and responsiveness, particularly for emerging technologies seeking rapid integration into clinical practice.
MARKET OPPORTUNITIES
Expansion of Telehealth and Remote Monitoring Solutions
One of the most promising opportunities for the Europe prenatal and neonatal equipment care market lies in the expansion of telehealth and remote monitoring solutions. The integration of digital health technologies into maternal and neonatal care has gained momentum, particularly following the acceleration of virtual healthcare adoption during the pandemic.
The shift has created a growing need for connected prenatal and neonatal equipment that enables remote data transmission and real-time monitoring.
Manufacturers are responding by developing wearable fetal monitors, mobile-compatible Doppler systems, and cloud-integrated neonatal vital sign trackers.
Also, home-based maternal monitoring kits are gaining traction, particularly among expectant mothers with limited access to urban medical centers. With continued investment in digital health infrastructure and supportive policy frameworks from the EU’s Digital Single Market initiative, the adoption of telehealth-enabled prenatal and neonatal equipment is expected to accelerate across the region.
Rising Focus on Maternal and Infant Homecare Settings
An emerging opportunity in the Europe prenatal and neonatal equipment care market is the growing interest in maternal and infant homecare settings. As healthcare systems seek to reduce hospital overcrowding and improve patient comfort, there is increasing adoption of portable and easy-to-use prenatal and neonatal monitoring devices for home use.
Similarly, neonatal homecare is becoming more prevalent for stable preterm infants requiring ongoing respiratory support or temperature regulation. Devices such as home phototherapy units, transport incubators, and apnea monitors are increasingly being deployed under physician supervision, supported by home nursing services and telehealth consultations.
Moreover, consumer preference for personalized, family-centered care models is driving demand for user-friendly neonatal equipment suitable for home environments. With government-backed initiatives promoting decentralized healthcare delivery and private insurers expanding coverage for homecare services, the market for prenatal and neonatal equipment in home settings is poised for significant growth, offering manufacturers a compelling avenue for expansion.
MARKET CHALLENGES
Shortage of Specialized Healthcare Professionals and Training Gaps
One of the foremost challenges facing the Europe prenatal and neonatal equipment care market is the shortage of specialized healthcare professionals trained to operate advanced diagnostic and life-support equipment. As medical technology becomes more sophisticated, the demand for skilled neonatologists, obstetricians, and pediatric nurses has intensified, yet workforce shortages persist in many regions.
Moreover, training gaps in the use of modern diagnostic and monitoring systems hinder optimal deployment. The situation is further complicated by aging healthcare workforces and declining recruitment in neonatology and maternal-fetal medicine specialties. As per a 2024 white paper by the Royal College of Midwives, over 30% of neonatal nurses in the UK planned to retire within the next five years , exacerbating staffing pressures.
To address this issue, governments and industry players are investing in simulation-based training and digital learning modules. However, until workforce planning and professional development programs align with technological advancements, skill shortages will continue to impede market growth.
Limited Access to Advanced Equipment in Rural and Low-Income Regions
A persistent challenge in the Europe prenatal and neonatal equipment care market is the disparity in access to advanced care technologies in rural and low-income regions. While urban hospitals in Germany, France, and the UK benefit from state-of-the-art neonatal intensive care units (NICUs) and digital prenatal monitoring systems, many rural and economically disadvantaged areas struggle with outdated or insufficient equipment.
This gap is exacerbated by uneven distribution of healthcare funding and infrastructure.
Additionally, the absence of standardized procurement policies across EU member states contributes to inconsistent device availability. A 2024 report by the European Association of Perinatal Medicine noted that countries like Bulgaria and Romania had less than one neonatal ventilator per 10,000 live births , compared to three to four in Western Europe.
Addressing these disparities requires targeted investments, cross-border collaboration, and stronger coordination between national health ministries and medical equipment suppliers to ensure equitable access to life-saving prenatal and neonatal technologies across the continent.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
6.25 % |
|
Segments Covered |
By Product and Country. |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis; DROC, PESTLE Analysis, Porter's Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
UK, Germany, Italy, France, Spain, Sweden, Denmark, Poland, Switzerland, Netherlands, Rest of Europe. |
|
Market Leader Profiled |
CareFusion Corporation, Covidien PLC, Drägerwerk AG & Co. KGaA |
SEGMENTAL ANALYSIS
By Product Type Insights
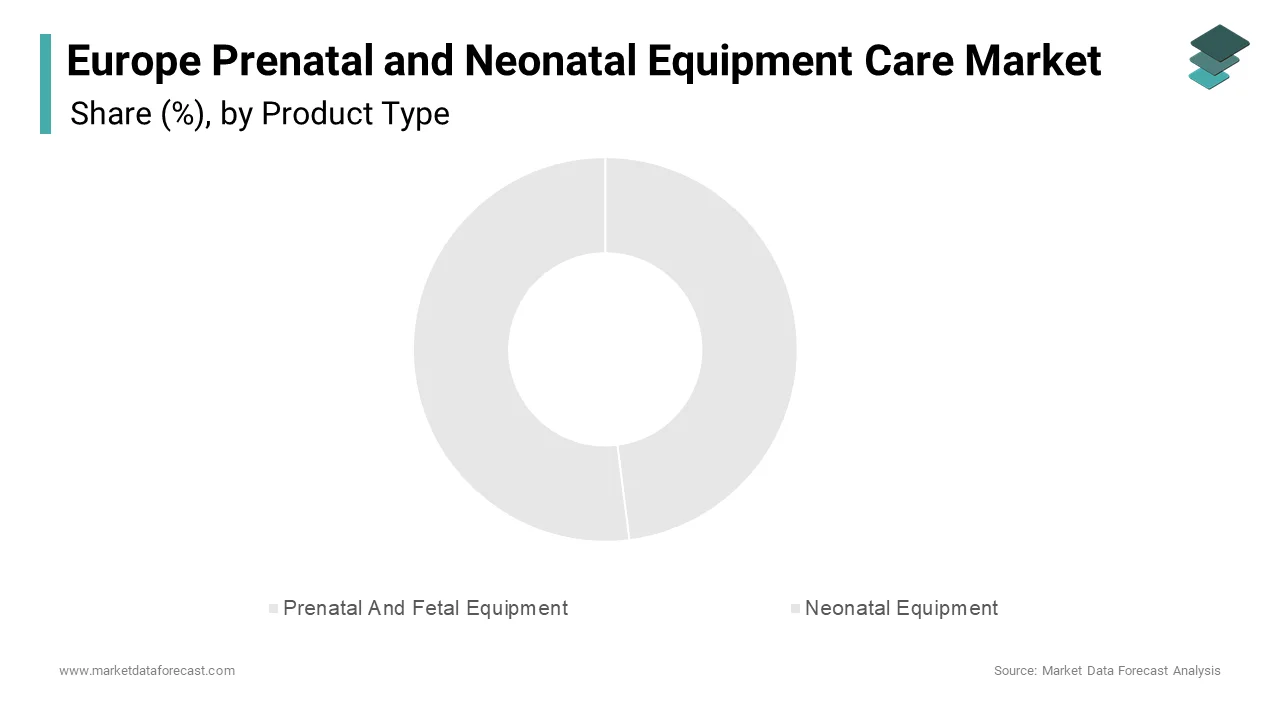
The neonatal equipment segment dominated the Europe prenatal and neonatal equipment care market, capturing 48.2% of total revenue in 2024. This leading position is attributed to the high demand for life-supporting and monitoring devices for newborns, particularly preterm infants requiring intensive care immediately after birth.
Germany and France, being among the most populous EU nations, contribute significantly to this demand.
Also, the rising prevalence of neonatal jaundice has increased the usage of phototherapy units. With continued advancements in neonatal care and growing emphasis on early intervention, the neonatal equipment segment remains the largest contributor to the European market, driven by clinical necessity, technological innovation, and increasing healthcare investments in infant health outcomes.
The prenatal and fetal equipment segment is currently the fastest-growing in the Europe prenatal and neonatal equipment care market, projected to expand at a CAGR of 9.7%. This rapid growth is fueled by increasing awareness around maternal health, rising cases of high-risk pregnancies, and advancements in diagnostic and monitoring technologies.
Moreover, non-invasive prenatal testing (NIPT) and advanced ultrasound imaging technologies are gaining traction across major European markets.
Companies like Philips Healthcare, GE Healthcare, and Siemens Healthineers are introducing cloud-connected fetal monitoring platforms that enable remote access to maternal and fetal health data.
Furthermore, portable Doppler and handheld fetal monitors are expanding into homecare settings, especially for expectant mothers with limited access to urban medical centers. With rising investment in maternal telehealth and early risk identification, the prenatal and fetal equipment segment is poised for sustained growth across the region.
COUNTRY LEVEL ANALYSIS
Germany held the largest market share in the Europe prenatal and neonatal equipment care market, contributing 19.1% of total regional revenue in 2024. The country’s strong healthcare infrastructure, high standards of maternal and child health, and significant public funding for perinatal services underpin its dominant position.
Germany's robust network of Level III neonatal intensive care units (NICUs) ensures access to life-saving equipment such as ventilators, incubators, and phototherapy systems, particularly for preterm infants.
The German government has also been proactive in integrating digital health technologies into maternal and neonatal care. As per the Federal Ministry of Health, over 85% of large maternity clinics in Germany adopted cloud-connected fetal monitoring systems in 2023 , enabling remote diagnostics and centralized patient management.
In addition, Germany is a hub for leading manufacturers such as Drägerwerk AG and Siemens Healthineers, which continuously introduce advanced diagnostic and therapeutic solutions tailored for European healthcare needs. With a strong regulatory framework, ongoing R&D initiatives, and a focus on reducing neonatal mortality, Germany remains at the forefront of the European prenatal and neonatal equipment care market.
The United Kingdom is maintaining a strong presence due to its well-established National Health Service (NHS) and progressive maternal healthcare policies. The NHS has prioritized early diagnosis and continuous monitoring of high-risk pregnancies, driving consistent demand for advanced prenatal and neonatal devices.
Additionally, the NHS Long Term Plan emphasizes neonatal care improvements, particularly in neonatal transport and home-based follow-up care.
Private sector involvement is also growing, with companies like Philips and Medtronic investing in specialized training programs for midwives and neonatal nurses. With strong policy backing, increasing private-public collaboration, and a focus on reducing maternal and neonatal mortality, the UK continues to be a key player in shaping the European prenatal and neonatal equipment landscape.
France is distinguished by its strong emphasis on maternal diagnostics and preventive care. The country has seen a surge in the adoption of advanced imaging systems, non-invasive prenatal testing (NIPT), and AI-driven fetal monitoring, reflecting broader trends toward precision medicine.
This trend has reinforced the need for enhanced prenatal diagnostics, prompting widespread integration of 3D/4D ultrasounds and Doppler flow assessments in maternity wards.
Also, France’s participation in EU-funded projects such as NeoGrowth and ePregnancy has accelerated the deployment of connected maternal health technologies. Public-private partnerships between academic institutions and medical device manufacturers are further strengthening the market. With a mature regulatory environment, growing awareness of prenatal risks, and a strong base of research institutions, France is emerging as a key driver of innovation and adoption in the European prenatal and neonatal equipment care sector.
Italy is leveraging its well-developed hospital system and growing emphasis on neonatal intensive care unit (NICU) modernization. The country has made significant strides in upgrading its NICUs, particularly in northern regions, where maternal and infant healthcare standards align closely with those of Western Europe.
Moreover, Italian hospitals have increasingly adopted advanced neonatal equipment, including microprocessor-controlled incubators, synchronized nasal intermittent positive pressure ventilation (SNIPPV) systems, and transcutaneous bilirubinometers.
Also, Italy is seeing a rise in home-based neonatal care models, supported by wearable apnea monitors and mobile-compatible phototherapy devices. With increasing government support, a strong focus on neonatal outcomes, and active engagement in EU-wide maternal health initiatives, Italy is steadily reinforcing its position in the European prenatal and neonatal equipment care market.
Spain is experiencing steady growth due to increasing investments in telemedicine-enabled prenatal diagnostics and neonatal monitoring. The country’s healthcare authorities have prioritized maternal-fetal telemonitoring, particularly in semi-urban and rural areas where specialist obstetricians may not always be available.
The expansion of digital health platforms has played a crucial role in this shift. In addition, Spain has been proactive in promoting home-based neonatal care for stable preterm infants. With continued government-backed digital health initiatives and increasing private-sector involvement, Spain is positioning itself as a key growth market for innovative prenatal and neonatal equipment in Europe.
KEY MARKET PLAYERS AND COMPETITIVE LANDSCAPE
Companies playing a significant role in the European prenatal and neonatal equipment care market profiled in this report are CareFusion Corporation, Covidien PLC, Drägerwerk AG & Co. KGaA, Cooper Surgical, Inc., FUJIFILM SonoSite, Inc., GE Healthcare, Getinge AB, Fisher & Paykel Healthcare Limited, Natus Medical Incorporated, Philips Healthcare, Siemens Healthcare, Nonin Medical, Inc., and Smiths Medical.
The competition in the Europe prenatal and neonatal equipment care market is intense, shaped by the presence of well-established global manufacturers, growing local players, and increasing demand for technologically advanced care solutions. Leading firms like Philips Healthcare, Drägerwerk AG, and GE Healthcare dominate due to their extensive product portfolios, strong R&D capabilities, and deep-rooted relationships with public and private healthcare institutions. These companies continuously refine their offerings to meet evolving clinical standards and regulatory requirements under the EU’s Medical Device Regulation (MDR).
At the same time, smaller European firms and emerging startups are challenging traditional players by introducing niche innovations, particularly in portable diagnostics, wearable sensors, and AI-assisted monitoring systems. Countries like Sweden and the Netherlands have become hotspots for digital health startups targeting maternal and neonatal care, further diversifying the competitive landscape.
Public procurement policies and cost containment measures also influence market dynamics, especially in Southern and Eastern Europe, where budget constraints favor refurbished or multi-functional equipment. Private-label and regional brands are gaining traction in these markets, offering more affordable alternatives without compromising essential functionalities.
Moreover, the rising emphasis on home-based maternal and neonatal care has prompted manufacturers to develop user-friendly, compact versions of traditional hospital-grade equipment. As the sector moves toward greater digitization, interoperability, and personalized monitoring, the battle for market share will increasingly hinge on technological differentiation, ease of integration, and strategic partnerships across the healthcare value chain.
Top Players in the Market
One of the leading players in the European prenatal and neonatal equipment care market is Philips Healthcare , a global innovator in medical technologies. The company offers a comprehensive portfolio of fetal and neonatal monitoring systems, including cloud-connected CTG devices, maternal-fetal monitors, and advanced NICU solutions. Philips plays a crucial role in shaping clinical workflows through its integrated diagnostic platforms, which are widely adopted across major hospitals in Germany, France, and the UK.
Another key player is Drägerwerk AG , a German manufacturer known for its high-precision neonatal ventilators, incubators, and patient monitoring systems. Dräger has been instrumental in advancing respiratory support technologies for preterm infants, with a strong presence in Level III neonatal intensive care units across Europe. Its commitment to innovation and compliance with stringent EU medical device regulations has made it a trusted name in critical perinatal care.
GE Healthcare also holds a prominent position in the market, offering a wide range of prenatal imaging systems such as Voluson ultrasound platforms that are extensively used for fetal diagnostics. GE's focus on AI-enabled imaging analytics and remote monitoring solutions has enhanced early detection of congenital anomalies, reinforcing its influence in both hospital and homecare settings across Europe.
Top Strategies Used by Key Market Participants
A primary strategy employed by leading companies in the Europe prenatal and neonatal equipment care market is continuous product innovation and digital integration . Firms are investing heavily in AI-driven diagnostics, wearable monitoring devices, and cloud-based data platforms to enhance accuracy, improve clinical decision-making, and support telemedicine applications in maternal and neonatal care.
Another major approach is strategic collaborations with academic institutions and healthcare providers . By partnering with universities, research centers, and maternity clinics, companies gain valuable insights into evolving clinical needs and accelerate the development of next-generation equipment tailored to regional healthcare demands.
Lastly, industry leaders are focusing on expanding their service networks and training programs to ensure proper usage and maintenance of complex neonatal and prenatal devices. Through technical workshops, simulation labs, and digital learning modules, manufacturers are enhancing user confidence among clinicians and midwives, thereby improving adoption rates and long-term customer loyalty in the competitive European market.
MARKET SEGMENTATION
This research report on the europe prenatal and neonatal equipment care market has been segmented and sub-segmented into the following categories.
By Product Type
- Prenatal And Fetal Equipment
- Neonatal Equipment
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Frequently Asked Questions
Is there government support for neonatal care in Europe?
Yes, many European governments and health agencies support neonatal care through national health systems, funding, and neonatal screening and prevention programs.
Which countries in Europe contribute most to the market?
Major contributors include Germany, France, the UK, Italy, and Spain, owing to advanced healthcare systems, high neonatal care standards, and significant investment in hospital infrastructure.
What are the main drivers of market growth in Europe?
Key drivers include rising preterm birth rates, increased awareness of neonatal health, advancements in healthcare infrastructure, and favorable government policies for maternal and infant care.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
