Middle East & Africa Monoclonal Antibodies Market Size, Share, Trends & Growth Forecast Report By Source, Indication, End-User, Application and Country (KSA, UAE, Israel, rest of GCC countries, South Africa, Ethiopia, Kenya, Egypt, Sudan, rest of MEA), Industry Analysis From 2025 to 2033
Middle East & Africa Monoclonal Antibodies Market Size
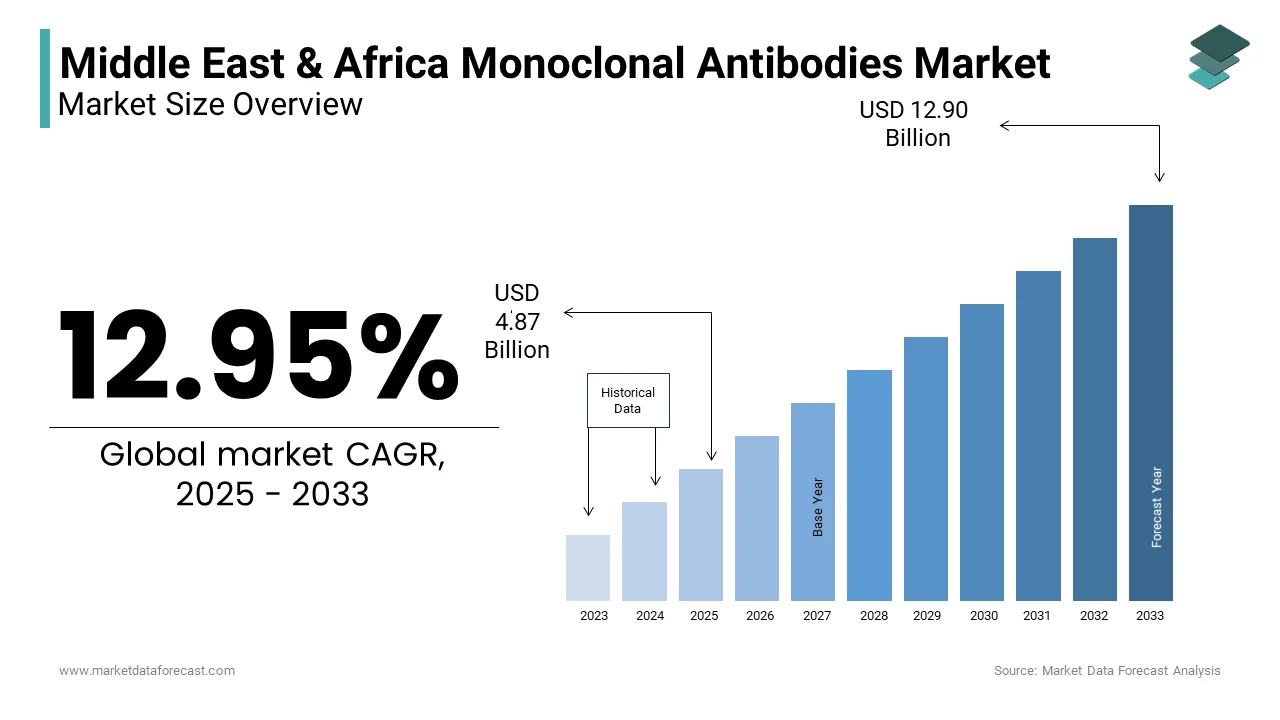
The monoclonal antibodies market size in the Middle East and Africa was worth USD 4.31 Billion in 2024. The regional market is estimated to be growing at a CAGR of 12.95% from 2025 to 2033 and be worth USD 12.90 billion by 2033 from USD 4.87 billion in 2025.
Monoclonal is a class of antibodies with the identical progeny of a hybridoma, derived by cell division from a single ancestral cell.
MARKET DRIVERS
The growing demand for personalized medicine is a vital factor responsible for developing therapeutic antibodies to provide targeted therapies, as each individual responds differently to a particular treatment. Furthermore, the advantages associated with using monoclonal antibodies for therapeutic purposes, including reducing adverse effects, homogeneity, specificity, and large-scale production, contribute to significant market growth. Moreover, increasing research collaborations to develop a strong drug portfolio is an important factor that is expected to drive the MEA monoclonal antibodies market growth during the forecast period.
In addition, government initiatives aimed at enabling the cost-effective production of monoclonal antibodies are expected to foster the emergence of this sector during the forecast period. For example, the National Institute of Standards and Technology's Biologic Manufacturing Initiative provides scientifically sound regulatory guidance to ensure the efficient and safe manufacture of protein therapies and help biopharmaceutical manufacturers deliver high-quality protein drugs for a low-cost region-wide. In addition, technological advancements in immunoassay offer a growth opportunity in the market.
The growing number of coronavirus cases increases the need for precise treatment options, leading to the development of monoclonal antibodies and, therefore, would help the monoclonal antibody market grow significantly in the years to come. Unlimited production and identification of specific clones against a particular antigen make this a unique product designed with ease in laboratories.
MARKET RESTRAINTS
A disadvantage of drugs formulated from monoclonal antibodies is that they are particular in nature and have limited purposes. This is because they interact with cells and do not penetrate them. To some extent, this has hampered the global monoclonal antibody market. Another difficulty in using monoclonal antibodies is that they must be injected, unlike small molecule drugs. The growth of the MEA monoclonal antibodies market would be challenged by the high cost involved in developing therapeutic monoclonal antibodies, the threats associated with counterfeit drugs, and the legal regulations required for the identification of molecules, which are limiting the growth of the market. The production and maintenance of infrastructure are expensive and often difficult, especially in low-income, underdeveloped countries.
REGIONAL ANALYSIS
Saudi Arabia is the most dominant regional market in the monoclonal antibody therapeutics market in the Middle East and Africa. It can be attributed to the presence of a well-established healthcare infrastructure. In addition, its large patient population base, well-established reimbursement policies, high disease awareness, government support for infection control and management, increasing incidence of lifestyle-associated diseases, and increased investment in R&D activities by the government for cancer contribute to the growth of Middle Eastern participation.
The United Arab Emirates monoclonal antibodies market is predicted to showcase a lucrative growth rate. The market is growing due to the significant growth in the life sciences industry and the availability of funds from the government and non-government sectors for clinical diagnostics.
KEY MARKET PLAYERS
GlaxoSmithKline plc, Novartis AG, Pfizer Inc., Thermo Fisher Scientific Inc., Eli Lilly and Company, Seattle Genetics, Bristol-Myers Squibb, F. Hoffmann-La Roche Ltd., and Biogen Inc. are expected to account for the majority of the MEA Monoclonal Antibodies Market share during the forecast period.
MARKET SEGMENTATION
This research report on the Middle East & Africa monoclonal antibodies market is segmented & sub-segmented into the following categories.
By Source
- Murine
- Chimeric
- Humanized
- Human
By Indication
- Cancer
- Blood Cancer
- Breast Cancer
- Colorectal Cancer
- Lung Cancer
- Pancreatic Cancer
- Others
- Autoimmune Diseases
- Infectious Diseases
- Cardiovascular Diseases
- CNS Disorders
- Others (Inflammatory, Microbial Diseases, & Other)
By End-User
- Hospitals/Clinics
- Research Institute
- Diagnostic Laboratories
By Application
- Medical
- Experimental
- Western Blot
- ELISA
- Radioimmune Assays
- Immunofluorescence
- Others
By Country
- KSA
- UAE
- Israel
- Rest of GCC countries
- South Africa
- Ethiopia
- Kenya
- Egypt
- Sudan
- Rest of MEA
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 1600
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
