Asia-Pacific CRO Services Market Size, Share, Trends & Growth Forecast Report By Type, Therapeutic Area, End User & Country (India, China, Japan, South Korea, Australia, New Zealand, Thailand, Malaysia, Vietnam, Philippines, Indonesia, Singapore and Rest of Asia-Pacific), Industry Analysis From 2025 to 2033
Asia-Pacific CRO Services Market Size
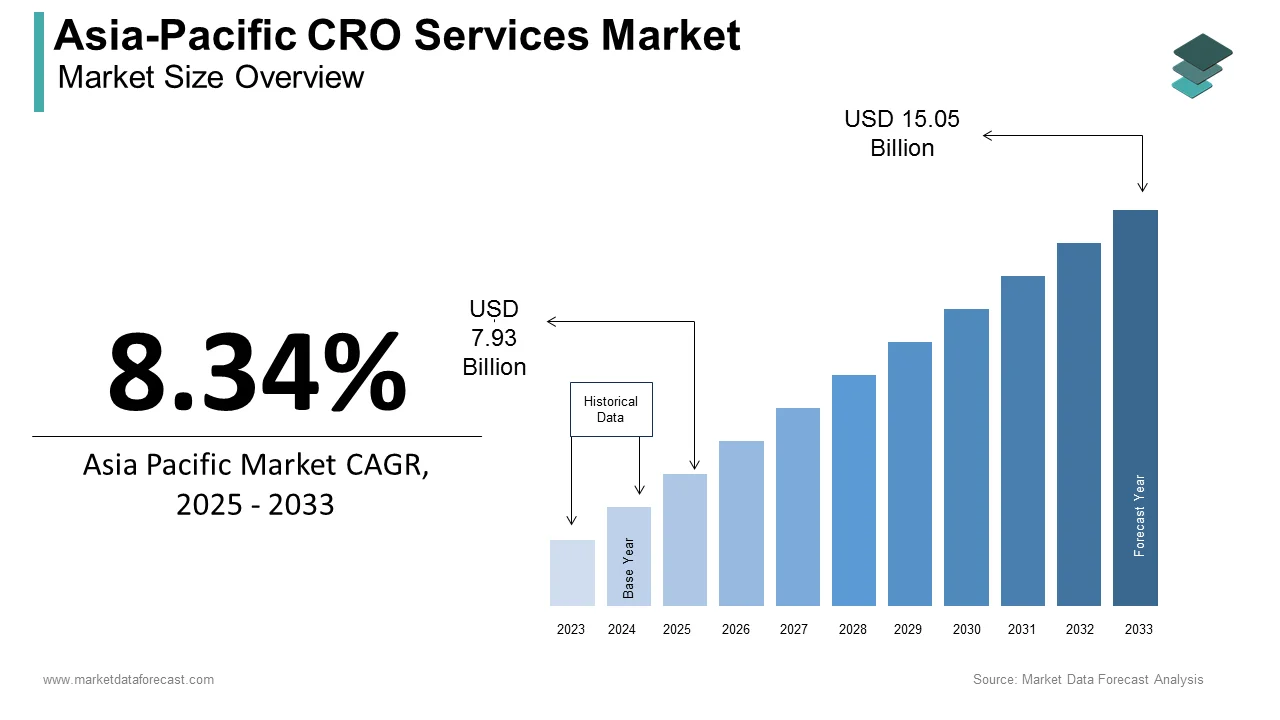
The contract research organization (CRO) services market size in Asia-Pacific was valued at USD 7.32 billion in 2024. The regional market is estimated to be worth USD 7.93 billion in 2025 and is projected to reach a valuation of USD 15.05 billion by 2033. it is predicted to register a CAGR of 8.34% from 2025 to 2033.
MARKET DRIVERS
The contract research organization services market in the Asia-Pacific region is projected to be fuelled by an increase in diseases such as hepatitis, stroke, and lung cancer. Also, the increasing number of clinical trials is uplifting the market growth. In addition, the proliferation of various diseases and rising dependence on contract research organizations are boosting market growth. Furthermore, the ease of regulatory compliance and the presence of leading clinical institutions contribute to Asia-new Pacific's status as a preferred destination for clinical trials. Increasing healthcare expenditure, rising awareness among the people, and rising prevalence of orphan and rare diseases are expected to drive the market growth during the forecast period.
Due to the significant risk of clinical trial failure, pharmaceutical and biopharmaceutical companies are likely to turn to CROs to outsource their R&D. Increasing investment for outsourcing research and development and rising demand for outsourcing analytical testing and clinical trial services are uplifting the market growth. Besides that, the increasing investments by the key market players in various clinical and non-clinical research and development activities outsourced by many contracts and research services that aid in cost-effective options for development products are accelerating market growth. In addition, the major pharmaceutical, biopharmaceutical, and medical companies in the region are investing in the development of novel drugs and devices, which expands the market growth.
MARKET RESTRAINTS
Significant technological advancements and rising globalization in developing countries such as China and India influence various biotech and pharmaceutical companies to outsource their research and development to many contract research organizations. Therefore, outsourcing is a crucial part of the biopharmaceutical industry, especially during the clinical research and development stages (R&D). The shortage of skilled professionals in some of the APAC countries limits the market's growth and is likely to negatively impact the market growth in the coming years.
SEGMENT ANALYSIS
By Type Insights
REGIONAL ANALYSIS
Geographically, the market in the Asia-Pacific region is anticipated to grow the most in the coming years. It is primarily due to the swift growth in the pharmaceutical industry, favorable government policies, an increase in pharmaceutical companies establishing manufacturing facilities, and lower costs for clinical trials in the region. In addition, technological advancements in developing countries like India and China will further aid the market's growth. The Asia Pacific is one of the fastest-growing regions with a promising revenue share in the Contract Research Organization Services Market. The CRO market for pharmaceutical and biopharmaceutical firms is expected to grow significantly, owing to the rising prevalence of chronic diseases such as cancer, cardiac disease, neurological disease, and infectious disease. Furthermore, pharma and biotech companies are outsourcing clinical trials to create new vaccines and treatments, in addition to higher government funding. This might aid in the treatment and prevention of illnesses like COVID-19. As a result, rising demand for CRO services will help the industry grow. Many new service releases have been recorded as agreements, partnerships, and collaborations with various pharmaceutical firms and academic institutes to research and develop COVID-19 vaccines, medicines, and diagnostics throughout the APAC. Countries such as China, India, and Japan have augmented the APAC regional market growth. China dominated the CRO market and recorded a significant share over the forecast period. The market growth is attributed to the rising demand for effective and healthcare devices and Technological advancements in the healthcare sector. On the other hand, the Contract Research Organization Services Market is anticipated to witness a promising share in the coming years owing to the improving healthcare sector and support from the government.
KEY MARKET PLAYERS
Quintiles Transnational Holdings Inc., Laboratory Corporation of America Holdings, Pharmaceutical Product Development, PAREXEL International Corporation, ICON Plc, PRA Health Sciences Inc., InVentiv Health Inc., Charles River Laboratories International Inc., INC Research Holdings Inc., and Wuxi PharmaTech are some of the major players in the Asia-Pacific market.
MARKET SEGMENTATION
This research report on the Asia-Pacific CRO services market is segmented and sub-segmented into the following categories.
By Type
- Clinical Research Services
- Phase I Clinical Research Services
- Phase II Clinical Research Services
- Phase III Clinical Research Services
- Phase IV Clinical Research Services
- Early-Phase Development Services
- Discovery Studies
- Chemistry, Manufacturing, & Control (CMC)
- Preclinical Services
- Pharmacokinetics/Pharmacodynamics (PK/PD)
- Toxicology Testing Services
- Other Preclinical Services
- Laboratory Services
- Bioanalytical Testing Services
- Analytical Testing Services
- Physical Characterization
- Raw Material Testing
- Batch-Release Testing
- Stability Testing
- Other Analytical Testing Services
- Consulting Services
By Therapeutic Area
- Oncology
- CNS
- Cardiovascular
By End User
- Pharmaceutical
- Biopharmaceutical
- Medical Device Companies
By Country
- India
- China
- Japan
- South Korea
- Australia
- New Zealand
- Thailand
- Malaysia
- Vietnam
- Philippines
- Indonesia
- Singapore
- Rest of Asia-Pacific
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com