Global eClinical Solutions Market Size, Share, Trends & Growth Forecast Report By Product (EDC and CDMS, CTMS, Clinical Analytics Platform, RTMS, Clinical Data Integration Platform, eCOA, Safety Solutions, eTMF, RMIS and Others), Delivery Mode, Development Phase, End-User and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa), Industry Analysis From 2025 To 2033.
Global eClinical Solutions Market Size
The global eClinical solutions market was worth US$ 9.71 billion in 2024 and is anticipated to reach a valuation of US$ 29.65 billion by 2033 from US$ 11 billion in 2025, and it is predicted to register a CAGR of 13.2% during the forecast period 2025-2033.
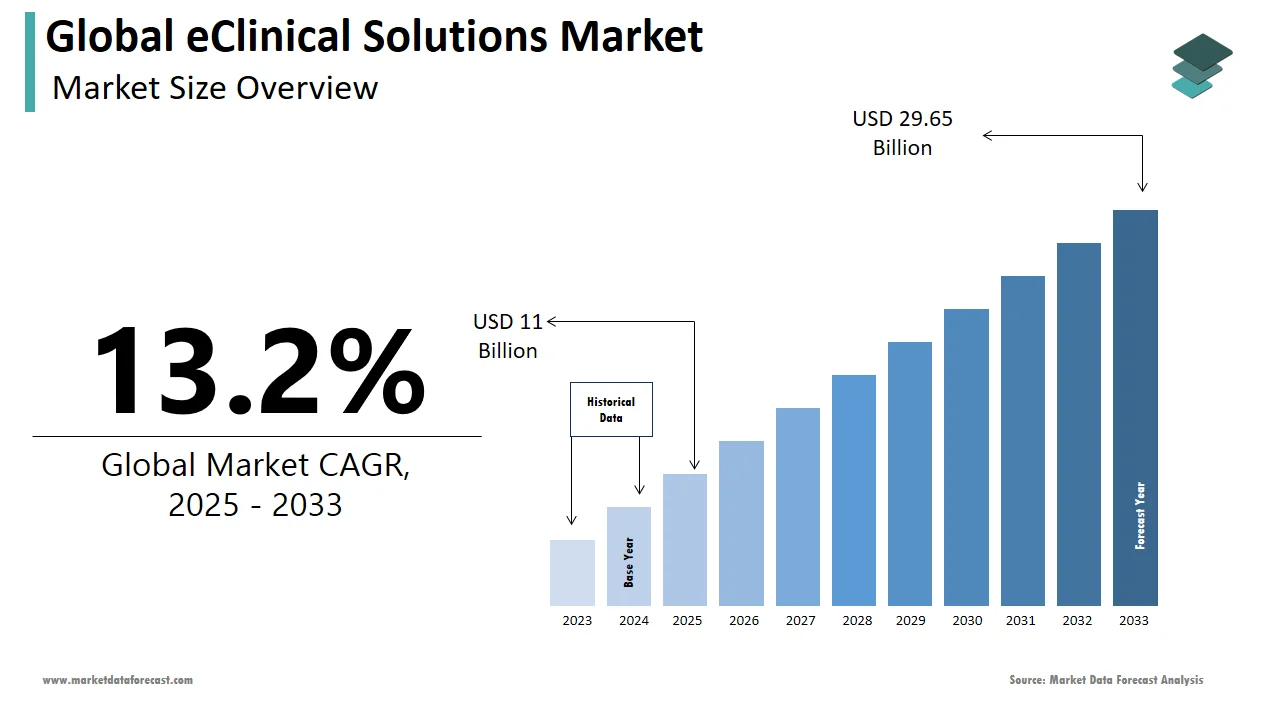
eClinical in clinical research is a word used to refer to electronic applications; they are used to remove manual, ad hoc, or paper-driven methods. eClinical technologies combine technology, products, and services to simplify the management and conduct of clinical trials. There are various types of eClinical technologies, such as Clinical Data Management Systems (CDMS), CTMS (Clinical Trials Management System), Randomization and Trial Supply Management Systems, e-PRO (Electronic Patient-Reported Outcome System), Electronic Trial Master File (eTMF), and other common forms of electronic solutions typically used in clinical trials.
Clinical trial data is expanding rapidly as clinical trials continue to grow in complexity and global reach. Therefore, it is essential to handle these data requirements. Implementing eClinical technology in a study helps us in several ways, such as real-time data access, clean data at any given time, an immediate resolution to queries, and locking patient data when clean. It is also possible to substantially reduce the Last Patient Last Visit - Database Lock timelines (i.e., from 8 to 10 weeks for paper studies to three to four weeks for an EDC.
MARKET DRIVERS
The growing usage of eClinical solutions in clinical trials, rising R&D activities, and increasing investments by various governments for clinical trials are propelling the global eClinical solutions market growth.
Pharmaceutical, life sciences, clinical research, and biopharmaceutical companies spend massive amounts on R&D to develop vaccines and drugs for various diseases such as diabetes, cancer, and AIDS. For instance, according to data published by the European Federation of Pharmaceutical Industries and Associations (EFPIA), the European pharmaceutical industry invested an estimated 39600 million Euros in 2020. In addition, the growing patient count suffering from chronic and lifestyle-related diseases and the increasing geriatric population worldwide are propelling the demand for eClinical solutions and boosting market growth. To address the growing burden of various diseases and the rising incidence among the aging population, pharmaceutical, and biopharmaceutical companies are conducting several drug discovery activities, leading to the growing usage of clinical trials and eClinical solutions. Countries like China, India, and Poland have become common destinations for clinical trials among pharmaceutical companies to have a large pool of patients and reduce costs.
In addition, low operating costs and transfer of resources are resulting in the growth of CRO companies, which is also expected to favor the eClinical solutions market, especially in APAC. Across the world, India, China, Taiwan, and Korea have become lucrative countries to outsource clinical trials as they are primarily populated, cost-effective, and developing economies. Furthermore, the growing adoption of cloud technologies to develop eClinical suits, increasing R&D activities in the clinical research segment, and untapped potential in developing countries are anticipated to offer lucrative growth possibilities to the market participants of the global eClinical solutions market.
MARKET RESTRAINTS
High costs associated with eClinical solutions are one of the key factors limiting the market's growth rate. In addition, budget constraints of some of the market participants, lack of standardization and interoperability issues, and reluctance from healthcare professionals towards the habituation of technological developments are anticipated to showcase a negative impact on the eClinical solutions market. Furthermore, the issues associated with data security and privacy are hindering the market growth. Issues with integration with existing healthcare systems, regulation, and compliance are further estimated to impede market growth.
Impact of COVID-19 on the Global eClinical Solutions Market
The COVID-19 pandemic created an inflection point for the eClinical solutions market. Most pharmaceutical and biotechnology companies have based their R&D activities on helping to improve the COVID-19 vaccine. Therefore, in the battle against COVID-19, several businesses have invested significant resources in using analytics. Historically, clinical trial tasks used to be carried out by traditional approaches such as 'face-to-face,' physical form filings, and so on, including recruitment, retention, implementation of treatments, and data collection.
Dramatic improvement has been observed in the COVID-19 pandemic regarding clinical trials with e-technologies. Researchers are now using electronic methods for clinical trial tasks, such as designing protocols, engaging with research workers, randomizing participants, collecting data, and reviewing outcomes. The COVID-19 pandemic is predicted to impact the region's eClinical Solutions production significantly. As a result of its high population and better control of the COVID-19 pandemic, countries across the region have earned the benefits of investing in eClinical solutions.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
13.2% |
|
Segments Covered |
By Product, Delivery Mode, Development Phase, End-User, and Region. |
|
Various Analyses Covered |
Global, Regional, and country-level analysis; Segment-Level Analysis, DROC; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape; Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
Oracle Corporation, Medidata Solutions, Inc., Parexel International Corporation, Bioclinica, Inc., Datatrak International, Inc., CRF Health, ERT Clinical, Merge Healthcare Incorporated, Omnicomm Systems, Inc., Maxisit Inc., Bio-Optronics, Inc., Eclinical Solutions, LLC., and Others. |
SEGMENTAL ANALYSIS
By Product Insights
Based on the product, the Electronic Clinical Outcome Assessment (eCOA) segment is expected to grow faster during the forecast period. This sub-segment helps maintain quality and is now incorporated with patient-reported, clinician-reported, and observer-reported outcomes. This method also helps in practical data analysis and thus boosts the market's growth. The increased number of patients suffering from various diseases is a major driving factor for this market. In addition, integrating patient care with clinical research studies is necessary for today's healthcare system and will boost this segment.
The CTMS segment accounted for the leading share of the market in 2024.
Segments such as Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS) had a substantial market share in 2024 and are expected to grow reasonably during the forecast period. The rise in the prevalence of diseases, the increasing geriatric population, and improving healthcare service standards will improve segment growth.
By Delivery Mode Insights
Based on delivery mode, the web-hosted segment accounted for the largest worldwide market share in 2024.
On the other hand, the cloud-based segment is estimated to grow at the highest CAGR in the global market during the forecast period.
By Development Phase Insights
Based on the development phase, the phase III segment had the largest worldwide market share in 2024.
By End-User Insights
Based on the end-user, the CROs segment had the largest market share in 2024 and is expected to grow the fastest during the forecast period. The growing interest of pharma companies to reduce costs is a significant factor, and the rising usage of eClinical solutions further supports segment growth. In addition, increased efficiency of services and enhanced productivity will positively impact the market.
Pharmaceutical and biotechnology companies are expected to grow at a reasonable rate. The companies use eClinical solutions during clinical trials. The increasing demand for drugs due to the growing population will increase clinical trials and positively impact the global market.
REGIONAL ANALYSIS
Geographically, the North American market dominates the global eClinical solutions market, followed by Europe in 2024. The domination of the North American region is likely to continue throughout the forecast period owing to the number of clinical trials being carried out in the North American region. In addition, the growing number of R&D activities resulting in the production and launch of new drugs, growing government grants for clinical trials, and the increasing prevalence of clinical trials are propelling the growth rate of the North American market. Furthermore, the increasing number of healthcare facilities and rising investments from governmental and non-governmental organizations in favor of eClinical solutions are expected to fuel the market's growth rate in North America. In addition, the growing patient population and increasing adoption of technological developments in treatment procedures are predicted to support market growth in North America. The U.S. had the largest share of the North American region in 2024, followed by Canada. Therefore, the U.S. eClinical solutions market is anticipated to showcase a promising CAGR during the forecast period.
The European eClinical solutions market had the second-largest share worldwide in 2024. During the forecast period, the European market is anticipated to have decent occupancy in the European market. The growing aging population and increasing prevalence of various diseases primarily drive the growth of the European market. In addition, rising government initiatives and high healthcare standards have positively impacted market growth.
The Asia Pacific eClinical solutions market is expected to see rapid growth in the global market in terms of CAGR. The rising involvement of emerging economies in clinical trials is expected to drive regional market growth. In addition, the increase in population in India and China has increased the demand for more drugs to treat more people. As a result, it has led to more trials, thus improving the market's growth.
The Latin American eClinical solutions market is anticipated to showcase a steady CAGR during the forecast period.
KEY MARKET PARTICIPANTS
Key companies dominating the global eClinical Solutions Market profiled in this report are Oracle Corporation, Medidata Solutions, Inc., Parexel International Corporation, Bioclinica, Inc., Datatrak International, Inc., CRF Health, ERT Clinical, Merge Healthcare Incorporated, Omnicomm Systems, Inc., Maxisit Inc., Bio-Optronics, Inc., and Eclinical Solutions, LLC.
The major players are actively involved in organic and inorganic growth strategies to attain a competitive edge in the industry. Etc.
RECENT MARKET HAPPENINGS
- In September 2020, EClinical Solutions revealed the stimulated acquisition of its flagship Elluminate Clinical Data Platform and its latest Ascend Clinical Data Services launch.
- In December 2019, Sofpromed offered TrialMaster, advanced clinical data management services with electronic data capture (EDC) system. Pharma and biotech companies are organizing phase I-IV clinical oncology trials to assist clinical data management services.
- In August 2019, TrialMax, an eClinical outcome assessment (eCOA) used for a trial promoted by a U.S. biotech company, partnered with CRF Health Phase-III prostate cancer. The partnership serves as a support in gathering clinical and endpoint data electronically.
- In October 2019, Datatrak International, Inc. announced their deliberation and appointments of EClinical Platform discussions, as it will be showcasing the Datatrak Enterprise Cloud at the 16th DIA Japan.
MARKET SEGMENTATION
This research report on the global eClinical solutions market has been segmented and sub-segmented based on the type, end-user, and region.
By Product
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platform
- Randomization and Trial Supply Management (RTMS)
- Clinical Data Integration Platform
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trial Master Files (eTMF)
- Regulatory Information Management Solutions (RIMS)
- Others
By Delivery Mode
- Web-hosted (On-demand)
- Licensed Enterprise (On-premises)
- Cloud-based
By Development Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-User
- Hospitals
- CROs
- Academic institutes
- Pharma & Biotech Organizations
- Medical Device Manufacturers
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
How much was the global eClinical solutions market worth in 2024?
The global eClinical solutions market size was valued at USD 9.71 billion in 2024.
What is the growth rate of the global eClinical solutions market?
The global eClinical solutions market is anticipated to grow at a CAGR of 13.2% from 2025 to 2033.
Which region held major share of the global eClinical solutions market in 2024?
North America captured the largest share of the worldwide market in 2024.
What are the companies playing a leading role in the eClinical solutions market?
Oracle Corporation, Medidata Solutions, Inc., Parexel International Corporation, Bioclinica, Inc., Datatrak International, Inc., CRF Health, ERT Clinical, Merge Healthcare Incorporated, Omnicomm Systems, Inc., Maxisit Inc., Bio-Optronics, Inc., and Eclinical Solutions, LLC. are some of the notable companies in the global eClinical solutions market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
