Europe Pharmacovigilance Market Size, Share, Trends & Growth Forecast Report By Clinical Trial Phase, Service Provider, Method & Country (UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic and Rest of Europe) - Industry Analysis, From (2025 to 2033)
Europe Pharmacovigilance Market Size
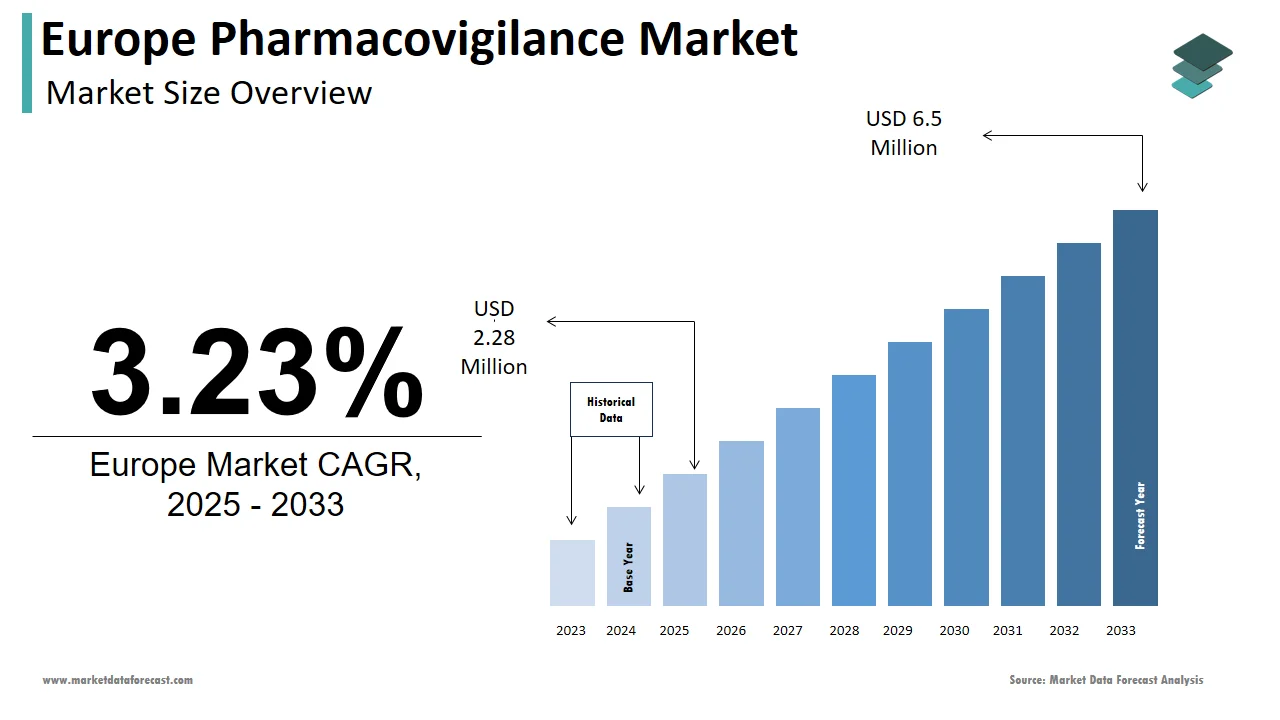
The pharmacovigilance market size in Europe was valued at USD 2 million in 2024. The European market is estimated to be worth USD 6.45 million by 2033 from USD 2.28 million in 2025, growing at a CAGR of 3.23% from 2025 to 2033.
MARKET SCENARIO
The European region had the second-largest market share of the global pharmacovigilance market, following North America. The EU has also developed a pharmacovigilance center called Eudra Vigilance to reduce the time required for clinical trials, boosting the market growth. There is a sizeable technological advancement in the field of pharmacovigilance, which is promoting the market growth. In addition, three biopharmaceutical companies in the region are doing drug research and development, leading to a rise in the number of clinical trials and are estimated to favor market growth.
The pharmacovigilance system consists of different technologies that are difficult to maintain without skilled persons. The lack of this specialized expertise is significantly hindering the market's growth rate. There is a lack of technological innovation in pharmacovigilance, impeding the need for the market in Europe. Strict rules and regulations by the government in approving new drugs are also slowly degrading the market demand.
COUNTRY LEVEL ANALYSIS

In Europe, the UK pharmacovigilance market is growing at a significant rate. Around 6.5% of the cases admitted in the hospital in the UK are of an adverse drug reaction; thus, there is an excellent demand for pharmacovigilance in the UK. In addition, increasing awareness through social media is also a factor fuelling the market's growth rate.
The pharmacovigilance market in Germany is next in line after the United Kingdom shows a robust growth rate. Increasing incidences of chronic diseases and a rise in the demand for quality treatment services escalate the market's growth rate.
KEY MARKET PLAYERS
A few noteworthy companies operating in the European pharmacovigilance market profiled in this report are Boehringer Ingelheim, Accenture, Bristol-Myers Squibb, Covance, ICON, PAREXEL, Quintiles, United BioSource, Synowlwedge, and Cognizant Technology Solutions Corporation.
MARKET SEGMENTATION
This Europe pharmacovigilance market research report is segmented and sub-segmented into the following categories.
By Clinical Trial Phase
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
By Method
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]