Global Preclinical CRO Market Size, Share, Trends & Growth Forecast Report – Segmented By Service Type (Bioanalysis And DMPK Studies, Toxicology Testing And Other Preclinical Services), End User (Biopharmaceutical Companies, Government And Academic Institutes And Medical Device Companies) and Region (North America, Europe, Asia Pacific, Latin America, And Middle East & Africa) - Industry Analysis (2025 To 2033)
Global Preclinical CRO Market Size
In 2024, the global preclinical CRO market was valued at USD 4.46 billion and it is expected to reach USD 9.14 billion by 2033 from USD 4.83 billion in 2025, growing at a CAGR of 8.3% during the forecast period.
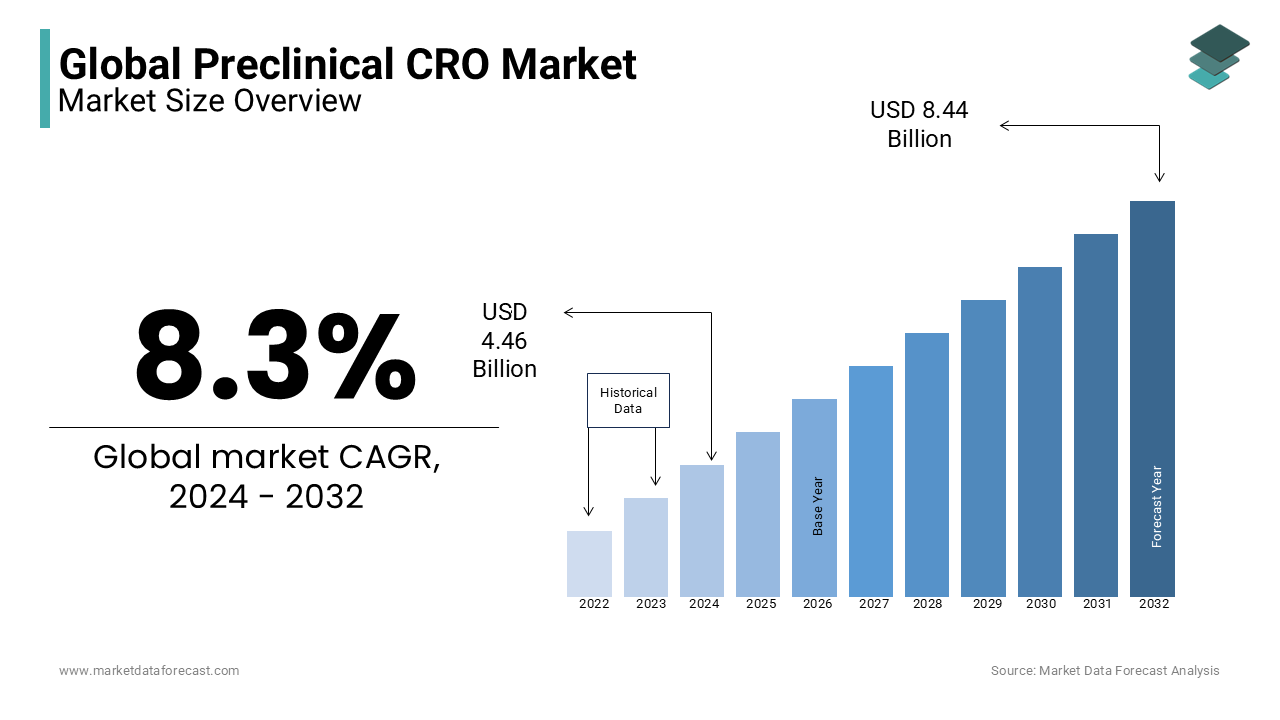
The global preclinical CRO market has experienced steady growth in the recent years. The growing complexity of drug development, stringent regulatory requirements and the pursuit of cost-efficiency have been increasing the demand for preclinical CRO in several countries and this trend is predicted to accelerate in the coming years and fuel the growth rate of the global preclinical CRO market. The United States, China, India and various European nations are the largest consumers of preclinical CRO services. The emphasis on outsourcing non-core activities to specialized service providers by pharmaceutical and biotechnological companies is gradually growing in several countries and is leading to the increasing demand for preclinical CRO services. In recent years, an increase in the number of strategic partnerships and collaborations between pharmaceutical companies and preclinical CROs has also been noticed in the global preclinical CRO market. As the globalization of clinical trials and demand for biopharmaceuticals increases further in the coming days, the preclinical CRO market registers new heights in terms of growth.
MARKET DRIVERS
The latest interest of key market players in the field is supporting the preclinical CRO market growth.
There has been continuous growth in the preclinical CRO market due to the growing investments from key market players. For example, Albert Labs International Corp. signs a Letter of Intent (LOI) with a full-service Contract-Research Organization (CRO), iNGEN, to conduct the Company's first-in-human study of its leading drug target, KRN-101. Albert Labs International Corp. is a pharmaceutical drug development company focused on obtaining regulatory approval for novel medications to treat various mental health conditions. RespireRx Pharmaceuticals Inc., a pioneer in the development of novel and ground-breaking therapies to treat illnesses brought on by disruption of neuronal signaling, is happy to announce that on November 14, 2022, the Company signed a letter of intent ("LOI") with a specialized clinical research organization with Australian headquarters that specializes in cannabinoid and psychedelic clinical research.
Y-O-Y growth in drug development pipelines, increasing outsourcing of preclinical research by pharmaceutical companies and growing patient population of chronic diseases worldwide are fueling the need for preclinical CRO and driving the global market growth. The rising demand for specialized preclinical services, increasing need for faster drug development timelines, growing focus on personalized medicine and technological advancements such as in vitro assays are supporting the growth rate of the global market.
MARKET RESTRAINTS
The regulations by animal rights protectors and animal testing restrictions are the major factors restraining the global preclinical CRO market.
For instance, in Europe, animal-based products or medicines are banned. Moreover, the rise in adopting alternative methods, such as micro-dosing hinders market growth. The preclinical CROs do not comply with international regulatory requirements like Good Laboratory Practice (GLP) standards, leading to quality issues, which is a major market challenge.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
CAGR |
8.3% |
|
Segments Analysed |
By Service Type, By End User |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regions Analysed |
North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
|
Key Market Players |
Charles River Laboratories International, Inc., Laboratory Corporation of America, Envigo, Eurofins Scientific, PRA Health Science, Inc., |
SEGMENTAL ANALYSIS
By Service Type Insights
The toxicology testing segment holds the largest market share in the preclinical CRO market based on service type. The rise in demand for new drugs and chemicals is boosting the market. Moreover, the growth in the outsourcing of non-core preclinical CRO studies and the adoption of toxicology tests fuel the market.
By End-User Insights
The biopharmaceutical segment had the largest share of the global preclinical CRO market ion 2023 and is predicted to register a healthy CAGR during the forecast period. Biopharmaceutical companies are the most significant end-user segment for preclinical CRO. The rise in outsourcing end-to-end services for pharmaceutical companies, primarily for small and mid-size companies, escalates the growth rate of the biopharmaceutical segment. In addition, the growth in drug entities based on rising competition is contributing to the expansion of the segment.
REGIONAL ANALYSIS
Geographically, North America dominated the preclinical CRO market in 2023 and the lead of the North American region in the global market is anticipated to continue throughout the forecast period. The presence of a massive number of preclinical market players is the main reason North America's market growth. The key North American market contributors by the United States and Canada. According to ClinicalTrail.gov, In 2020, approximately 45,400 preclinical studies were registered only in the United States. Further, pharmaceutical companies are interested in novel drug development for better treatment of chronic diseases, leading to North American market growth.
Asia-Pacific is predominantly rising during the forecast period. As a result, the size of the Asia Pacific preclinical CRO market was valued at USD 0.7 billion in 2020. India, China, and Japan are leading countries contributing to the Asia Pacific market share. The presence of numerous pharmaceutical companies in countries like India and China is the primary factor for market growth. Moreover, the increase in R&D investments and the secure government policies propels the Asia Pacific preclinical CRO market.
KEY MARKET PLAYERS
Some of the major companies dominating the global preclinical CRO market profiled in the report are Charles River Laboratories International, Inc., Laboratory Corporation of America, Envigo, Eurofins Scientific, PRA Health Science, Inc., Wuxi AppTec, Medpace, Inc., Pharmaceutical Product Development, LLC and Paraxel International Corporation.
RECENT HAPPENINGS IN THIS MARKET
- In January 2024, Oncodesign Services acquired ZoBio to boost its expertise and capabilities in the field of small molecule discovery. Oncodesign Services is a company that has CRO expertise in drug discovery & preclinical services and ZoBio is a CRO that has specialized in Small Molecule Drug Discovery.
- In November 2023, CEBIS International, a Europe-based CRO company, announced its expansion in the U.S. market.
- In July 2023, TransCure bioServices established a strategic collaboration with Preclina Inc. to increase their presence in the Asia-Pacific region and expand their services portfolio.
- In June 2023, PsychoGenics launched their AI Platform called eCube®.
MARKET SEGMENTATION
This research report on the global preclinical CRO market has been segmented and sub-segmented based on service type, end-user, and region.
By Service Type
- Bioanalysis And DMPK Studies
- Toxicology Testing
- Other Preclinical Services
By End-user
- Biopharmaceutical Companies
- Government And Academic Institutes
- Medical Device Companies
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
Frequently Asked Questions
How much is the global preclinical CRO market going to be worth by 2033 ?
As per our research report, the global preclinical CRO market size is projected to be USD 9.14 billion by 2033.
Which region is anticipated to witness considerable growth in the preclinical CRO market?
Geographically, the APAC preclinical CRO market is predicted to have the fastest growth rate in the global market from 2024 to 2033.
Who are the key players operating in the preclinical CRO market?
Charles River Laboratories International, Inc., Laboratory Corporation of America, Envigo, Eurofins Scientific, PRA Health Science, Inc., Wuxi AppTec, and Medpace, Inc. are some of the key players operating the preclinical CRO market.
At What CAGR, the global preclinical CRO market is expected to grow from 2024 to 2033?
The global preclinical CRO market is estimated to grow at a CAGR of 8.3% from 2024 to 2033.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
