North America DNA Sequencing Market Size, Share, Trends & Growth Forecast Report By Product Type (Instruments, Consumables, and Other Product Types), Sequencing Type (Sanger Sequencing, Next-Generation Sequencing, and Other Sequencing Types), Application (Diagnostics, Personalized Medicine, and Other Applications), End User (Hospitals and Healthcare Organizations, Academics and Research Institutions, Pharmaceutical and Biotechnology Companies, and Other End Users) and Country (United States, Canada, Mexico) Industry Analysis From 2025 to 2033.
North America DNA Sequencing Market Size
The size of the DNA sequencing market in North America was valued at USD 5.1 billion in 2024. This market is expected to grow at a CAGR of 12.2% from 2025 to 2033 and be worth USD 14.17 billion by 2033 from USD 5.72 billion in 2025.

The North America DNA sequencing market covers a wide range of technologies, services, and applications used to determine the precise order of nucleotides within a DNA molecule. This includes next-generation sequencing (NGS), Sanger sequencing, third-generation sequencing, and related bioinformatics tools that enable genomic analysis. The field plays a critical role in advancing precision medicine, cancer genomics, agricultural biotechnology, forensic science, and infectious disease research.
North America holds a dominant position in the global DNA sequencing landscape due to its strong research infrastructure, government funding for genomics initiatives, and presence of leading biotech and pharmaceutical companies. According to the National Institutes of Health (NIH), the U.S. alone accounted for a significant portion of global life sciences research expenditures in recent years, fostering innovation and adoption of advanced sequencing technologies. Moreover, increasing awareness about genetic disorders and rising demand for personalized medicine have accelerated the integration of DNA sequencing into clinical diagnostics.
MARKET DRIVERS
Advancements in Precision Medicine and Personalized Healthcare
One of the primary drivers of the North America DNA sequencing market is the rapid advancement of precision medicine and personalized healthcare approaches. Unlike traditional one-size-fits-all treatments, precision medicine leverages genomic insights to tailor therapies based on individual genetic profiles, significantly improving treatment outcomes for conditions such as cancer, cardiovascular diseases, and rare genetic disorders. According to the National Institutes of Health (NIH), the All of Us Research Program has enrolled over one million participants to build a diverse genetic database aimed at accelerating personalized medicine. This initiative has spurred increased collaboration between research institutions, hospitals, and sequencing service providers, expanding access to genomic testing in clinical settings. Furthermore, oncology remains a key application area, with targeted therapies increasingly reliant on tumor profiling through DNA sequencing. Like, approximately 30% of cancer patients now undergo some form of genomic testing before initiating treatment, highlighting the growing clinical relevance of sequencing technologies. These developments are supported by insurance coverage expansion, particularly in the U.S., where major payers like Medicare and private insurers are increasingly reimbursing genetic tests deemed medically necessary.
Expansion of Genomic Research Initiatives and Government Funding
Another key driver shaping the North America DNA sequencing market is the substantial investment in genomic research initiatives and federal funding support. Governments and research institutions across the region are prioritizing genomics as a cornerstone of future healthcare innovation, leading to sustained demand for high-throughput sequencing platforms and associated bioinformatics solutions. The National Human Genome Research Institute (NHGRI) reports that annual U.S. federal spending on genomics-related research has consistently exceeded $2 billion over the past decade, with additional contributions from private foundations and industry partners. Programs such as the NIH’s BRAIN Initiative and the Cancer Moonshot have further amplified the need for comprehensive DNA sequencing capabilities in neuroscience and oncology research. In parallel, Canada has launched national-level projects that focuses on integrating genomic data into public health strategies and rare disease diagnosis. Apart from these, academic institutions and hospital networks are investing in in-house sequencing labs to reduce turnaround times and enhance research agility.
MARKET RESTRAINTS
High Cost of Sequencing Equipment and Consumables
A significant restraint affecting the North America DNA sequencing market is the high cost associated with sequencing equipment, reagents, and consumables, which limits accessibility for smaller research institutions and community-based healthcare providers. Next-generation sequencing (NGS) platforms from leading manufacturers such as Illumina and Thermo Fisher Scientific often require capital investments exceeding hundreds of thousands of dollars, along with recurring expenses for chemical reagents, software licenses, and maintenance contracts. Like many university-affiliated laboratories face budget constraints that delay or prevent the acquisition of cutting-edge sequencing instruments, thereby slowing down research progress. Also, the complexity of NGS workflows necessitates specialized training and skilled personnel, adding to the overall operational costs. While larger academic medical centers and biopharmaceutical firms can absorb these expenses, independent clinics and regional hospitals struggle to integrate sequencing into routine diagnostics. The Centers for Disease Control and Prevention (CDC) notes that disparities in genomic testing access persist across different healthcare settings, limiting the scalability of precision medicine initiatives.
Data Management and Bioinformatics Complexity
Another critical challenge impeding the growth of the North America DNA sequencing market is the increasing complexity of data management and bioinformatics analysis. Modern sequencing technologies generate vast amounts of genomic data, requiring sophisticated storage solutions, computational power, and expert interpretation to derive meaningful clinical insights. According to the National Library of Medicine, a single whole-genome sequencing run can produce up to 2 terabytes of raw data, necessitating scalable cloud-based infrastructure and high-performance computing resources. Many healthcare institutions lack the necessary IT infrastructure or trained personnel to efficiently process, store, and analyze this information in real time. Moreover, standardization of genomic data formats and interoperability between different sequencing platforms remain ongoing challenges. The Office of the National Coordinator for Health Information Technology (ONC) highlights that inconsistent data integration practices hinder seamless exchange of genomic information between research labs, hospitals, and electronic health record (EHR) systems. As a result, even when sequencing is performed, extracting actionable insights often requires external bioinformatics support, increasing turnaround times and costs.
MARKET OPPORTUNITIES
Integration of AI and Machine Learning in Genomic Data Analysis
An emerging opportunity in the North America DNA sequencing market is the integration of artificial intelligence (AI) and machine learning (ML) in genomic data interpretation. As sequencing generates massive datasets, traditional manual analysis methods are proving inadequate to extract timely and accurate insights. AI-driven analytics offer a transformative solution by enabling faster pattern recognition, variant identification, and predictive modeling in clinical and research applications. Leading technology firms and bioinformatics startups are developing AI-powered platforms capable of analyzing complex genomic sequences with greater accuracy and efficiency. According to the National Institutes of Health (NIH), AI-assisted tools have improved diagnostic precision in rare disease detection by identifying previously undetectable mutations linked to inherited conditions. Besides, AI is playing a pivotal role in oncology, where it helps identify tumor-specific biomarkers and recommend targeted therapies based on a patient’s genomic profile.
Expansion of Population Genomics and Public Health Screening Programs
Another significant opportunity driving the North America DNA sequencing market is the growing emphasis on population genomics and large-scale public health screening initiatives. Governments and healthcare organizations are leveraging genomic data to understand disease prevalence, track genetic risk factors, and improve preventive healthcare strategies across diverse populations. The All of Us Research Program, spearheaded by the National Institutes of Health (NIH), aims to collect genetic information from over one million Americans to create a comprehensive health database tailored for precision medicine. Similarly, Canada’s national genomics strategy, led by Genome Canada, has expanded its focus on integrating genomic screening into newborn and carrier testing programs. Like, early genetic screening has proven effective in detecting rare metabolic disorders and enabling timely interventions, improving long-term health outcomes. In addition, state-level initiatives in the U.S., such as New York’s and California’s newborn screening expansions, are incorporating DNA sequencing to detect genetic conditions earlier in life.
MARKET CHALLENGES
Ethical, Legal, and Privacy Concerns Surrounding Genetic Data
A major challenge confronting the North America DNA sequencing market is the growing concern around ethical, legal, and privacy issues associated with genetic data collection and usage. As genomic testing becomes more integrated into healthcare and research, questions surrounding consent, data ownership, and potential misuse have intensified, prompting regulatory scrutiny and public debate. According to the National Human Genome Research Institute (NHGRI), concerns about genetic discrimination, particularly in employment and insurance,e are among the top barriers preventing individuals from participating in genomic studies. Despite protections under the Genetic Information Nondiscrimination Act (GINA), many consumers remain wary of how their genetic data may be used beyond clinical purposes. Moreover, the commercialization of direct-to-consumer (DTC) genetic testing services has raised concerns about data security and third-party sharing. The Federal Trade Commission (FTC) has issued warnings regarding the risks of unauthorized access and potential exploitation of genetic databases, especially when handled by non-medical entities. Regulatory frameworks are evolving to address these concerns, but inconsistencies across states and jurisdictions complicate compliance for sequencing providers.
Workforce Shortage and Lack of Skilled Professionals
Another pressing challenge facing the North American DNA sequencing market is the shortage of skilled professionals capable of operating advanced sequencing platforms and interpreting complex genomic data. While sequencing technologies have become more automated, the demand for qualified scientists, bioinformaticians, and clinical geneticists continues to outpace supply. According to the American Society of Human Genetics (ASHG), there is a significant gap between the number of available genomic professionals and the growing volume of sequencing requests in both research and clinical settings. Academic institutions report difficulty in training enough specialists to meet industry needs, particularly in bioinformatics and data interpretation. This workforce challenge is further compounded by the interdisciplinary nature of genomics, which requires expertise in molecular biology, computational analysis, and clinical genetics. The National Institutes of Health (NIH) emphasizes that expanding educational programs and professional certifications is essential to bridge this talent gap. Without adequate staffing, laboratories face delays in processing samples, hospitals encounter bottlenecks in diagnosing genetic conditions, and research institutions struggle to translate genomic discoveries into clinical applications.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Product Type, Sequencing Type, Application, End User, and Region. |
|
Various Analyses Covered |
Global, Regional and Country-Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Countries Covered |
India, China, Japan, South Korea, Australia, New Zealand, Thailand, Malaysia, Vietnam, Philippines, Indonesia, Singapore & Rest of APAC |
|
Market Leaders Profiled |
Agilent Technologies Inc., Bio-Rad Laboratories, Inc., Danaher, F. Hoffmann-La Roche Ltd, Illumina, Inc., Merck, Revvity, Thermo Fisher Scientific Inc., QIAGEN, Eurofins Genomics LLC, and others. |
SEGMENT ANALYSIS
By Product Type Insights
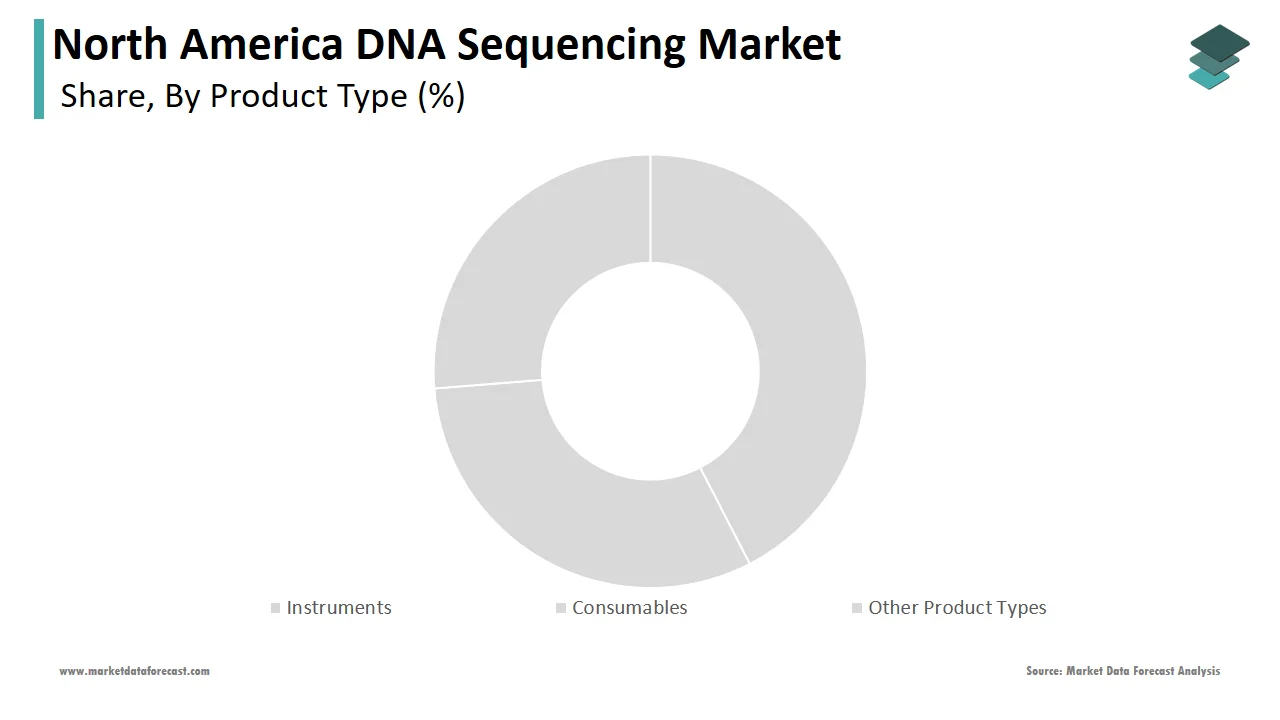
The consumables constituted the largest segment in the North America DNA sequencing market by capturing 52.1% of total revenue in 2024. This dominance is primarily attributed to the recurring nature of consumable purchases, which include reagents, kits, nucleotides, enzymes, and sample preparation materials essential for sequencing workflows. One of the key drivers behind this segment’s leadership is the high frequency of sequencing runs performed in research laboratories, clinical diagnostics centers, and biopharmaceutical companies. According to the National Institutes of Health (NIH), next-generation sequencing (NGS) platforms require continuous replenishment of specialized reagents, with each run consuming significant volumes of chemical compounds and enzymatic mixtures. Apart from these, the expansion of large-scale genomic initiatives such as the All of Us Research Program has significantly boosted demand for sequencing consumables across academic and healthcare institutions. Moreover, the increasing adoption of third-party reagent suppliers offering cost-effective alternatives to original equipment manufacturer (OEM) products has made consumables more accessible without compromising quality.
The instruments segment is the fastest-growing within the North America DNA sequencing market, projected to expand at a CAGR of 14.6%. This rapid surge is driven by continuous technological advancements, including the development of high-throughput sequencers, benchtop systems, and portable devices tailored for diverse applications ranging from clinical diagnostics to field research. A major factor fueling this expansion is the increasing deployment of next-generation sequencing (NGS) and third-generation sequencing (TGS) platforms in both academic and commercial settings. Furthermore, rising investments by sequencing technology providers in product innovation and automation have enhanced instrument performance while reducing operational complexity. Companies like Illumina, Thermo Fisher Scientific, and Pacific Biosciences are launching compact, cloud-integrated sequencing devices suited for decentralized testing environments, including hospital labs and point-of-care diagnostic centers. Also, government grants and private funding for genomics infrastructure have enabled smaller research institutions to acquire sequencing instruments they previously could not afford.
By Sequencing Type Insights
Next-Generation Sequencing (NGS) had the biggest share of the North America DNA sequencing market in 2024. This overwhelming dominance is due to NGS’s superior throughput, scalability, and cost-efficiency compared to traditional sequencing methods. A primary driver of NGS's leadership is its widespread adoption in clinical diagnostics, particularly in oncology, inherited disease screening, and infectious disease monitoring. According to the American Society of Clinical Oncology (ASCO), over 30% of cancer patients now undergo some form of genomic profiling using NGS to guide treatment decisions, underscoring its critical role in precision medicine. Additionally, the integration of NGS into population-wide genomics programs such as the NIH’s All of Us initiative has significantly expanded its application base. The National Library of Medicine reports that NGS usage in public health research has surged notably since 2020, driven by the ability to sequence entire genomes at unprecedented speeds. Moreover, NGS is increasingly being used in non-clinical fields such as agriculture, forensics, and environmental biology, broadening its market reach.
Third-generation sequencing technologies, classified under "Other Sequencing Types," represent the most rapidly expanding segment within the North America DNA sequencing market, anticipated to grow at a CAGR of 17.2%. This is fueled by the unique advantages offered by long-read sequencing platforms such as those developed by Pacific Biosciences and Oxford Nanopore Technologies. A key growth driver is the ability of these platforms to deliver real-time, single-molecule sequencing without the need for amplification, enabling direct detection of epigenetic modifications and structural variants that were previously difficult to capture using short-read NGS. According to the National Center for Biotechnology Information (NCBI), long-read sequencing has significantly improved genome assembly accuracy, making it indispensable for complex genomic regions. Also, third-generation sequencing is gaining traction in clinical microbiology and pathogen surveillance. As per the Centers for Disease Control and Prevention (CDC), real-time nanopore sequencing is now being deployed for outbreak tracking and antimicrobial resistance monitoring, enhancing public health response capabilities. Moreover, decreasing instrument costs and expanding applications in transcriptomics and metagenomics are accelerating adoption. Academic and industrial researchers are increasingly leveraging these platforms for de novo genome sequencing and RNA isoform identification, signaling strong growth potential for this segment in North America.
By Application Insights
The diagnostics segment dominated the North America DNA sequencing market by capturing an estimated 48.8% of total market revenue in 2024. This is attributed to the extensive use of DNA sequencing in identifying genetic mutations, diagnosing rare diseases, detecting cancer biomarkers, and monitoring infectious pathogens. One of the main factors driving this segment is the increasing integration of genomic testing into routine clinical diagnostics. Additionally, the expansion of newborn screening programs incorporating DNA sequencing has reinforced demand in early disease detection. As per the study, early diagnosis through genetic testing can reduce long-term healthcare costs by enabling timely interventions, making it a priority for both public and private healthcare providers. Furthermore, the rise in infectious disease outbreaks such as SARS-CoV-2 and antibiotic-resistant bacteria has spurred the use of sequencing for pathogen identification and genomic epidemiology.
Personalized medicine is the highest growing application segment in the North America DNA sequencing market, projected to expand at a CAGR of 19.4%. This quick growth is due to the increasing reliance on genetic data to tailor treatments based on individual genomic profiles, improving therapeutic outcomes in oncology, cardiology, and rare disease management. A key driver behind this surge is the expansion of pharmacogenomic testing, where DNA sequencing helps determine how individuals metabolize specific drugs. According to the National Institutes of Health (NIH), over 30 FDA-approved medications now carry pharmacogenomic labeling recommendations, encouraging physicians to incorporate sequencing into prescribing decisions. Apart from these, oncology remains a major growth engine, with personalized therapies increasingly dependent on tumor profiling. The American Cancer Society notes that targeted cancer treatments guided by genomic insights have significantly improved survival rates in certain malignancies, reinforcing the value of DNA sequencing in clinical decision-making. Moreover, direct-to-consumer (DTC) genetic testing companies are contributing to broader awareness and accessibility.
By End User Insights
The academic and research institutions represented the largest end-user segment in the North America DNA sequencing market by accounting for a 45.5% of total spending in 2024. This dominance is largely driven by extensive funding for genomics research, strong university-industry collaborations, and the presence of advanced sequencing facilities in major research universities and federal laboratories. A key growth driver is the sustained investment in national genomics initiatives. According to the National Institutes of Health (NIH), annual funding for genomic research exceeded $2.5 billion in 2023, supporting large-scale studies in population genetics, cancer biology, and neurogenomics. Programs such as the BRAIN Initiative and the All of Us Research Program have further amplified demand for sequencing services in academic settings. Besides, research institutions play a pivotal role in training the next generation of genomic scientists, requiring continuous procurement of sequencing equipment and consumables.
Pharmaceutical and biotechnology companies exhibit the most dynamic end-user segment in the North America DNA sequencing market, predicted to rise at a CAGR of 18.9%. This swift progress is driven by the increasing reliance on genomic data for drug discovery, target validation, and companion diagnostic development. A main aspect fueling this expansion is the shift toward precision oncology, where DNA sequencing plays a critical role in identifying patient-specific mutations for targeted therapies. According to the Personalized Medicine Coalition, over 40% of new drug approvals in recent years have included a genomic biomarker, highlighting the industry’s deepening integration of sequencing into therapeutic pipelines. Moreover, biotech firms engaged in gene therapy, CRISPR editing, and synthetic biology are increasingly utilizing sequencing to verify genetic constructs and monitor off-target effects. As per the Biotechnology Innovation Organization (BIO), the number of gene and cell therapy candidates in clinical trials has more than doubled since 2020, necessitating high-throughput sequencing capabilities. Also, partnerships between sequencing platform providers and pharmaceutical companies are accelerating the adoption of in-house sequencing labs for faster drug development cycles.
COUNTRY LEVEL ANALYSIS
The United States led with strong research infrastructure and clinical integration in the North America DNA sequencing market by holding a 88.7% of regional market share in 2024. This is attributed to the country’s robust life sciences ecosystem, extensive government funding for genomics research, and strong integration of DNA sequencing into clinical practice. A major growth driver is the presence of world-leading research institutions such as the National Institutes of Health (NIH), the Broad Institute, and the Jackson Laboratory, which are spearheading large-scale sequencing initiatives. The NIH reports that over one million participants have enrolled in the All of Us Research Program, generating vast genomic datasets that fuel innovation and downstream applications. According to the American Society of Clinical Oncology (ASCO), over 30% of cancer patients now receive treatment based on genomic profiling, underscoring the mainstream acceptance of DNA sequencing in precision medicine. Moreover, the presence of major sequencing technology developers such as Illumina, Thermo Fisher Scientific, and Pacific Biosciences has ensured continuous innovation and accessibility.
Canada is emerging as a strategic player due to its commitment to population genomics, government-backed research programs, and growing clinical adoption. A key growth factor is the Canadian Genomics Enterprise, led by Genome Canada, which funds large-scale research projects focused on rare diseases, indigenous health, and precision medicine. Additionally, provincial health authorities are integrating genomic testing into public healthcare systems, particularly for prenatal screening and cancer diagnostics. Like, newborn screening programs are expanding to include DNA sequencing for metabolic and neurological disorders, improving early intervention and treatment planning. Moreover, Canada has become an attractive location for international biotech firms seeking collaborative research opportunities and access to diverse genomic databases.
The Rest of North America, encompassing Mexico and select Caribbean territories, have a limited but developing market with strategic potential. Though currently a minor contributor, this segment exhibits early-stage growth potential due to expanding healthcare access, increasing research funding, and rising awareness about genomic medicine. Mexico, in particular, is emerging as a hub for outsourced genomics research and contract sequencing services. Additionally, public health initiatives are gaining traction, with pilot programs exploring DNA sequencing for maternal-fetal screening and rare disease diagnosis. However, challenges such as limited reimbursement policies, inconsistent regulatory frameworks, and lower adoption rates among hospitals continue to restrict large-scale commercialization.
KEY MARKET PLAYERS
A few of the notable companies operating in the North America DNA sequencing market profiled in this report are Agilent Technologies Inc., Bio-Rad Laboratories, Inc., Danaher, F. Hoffmann-La Roche Ltd, Illumina, Inc., Merck, Revvity, Thermo Fisher Scientific Inc., QIAGEN, Eurofins Genomics LLC and others.
TOP LEADING PLAYERS IN THE MARKET
Illumina, Inc.
Illumina is a global leader in DNA sequencing technologies and holds a dominant position in the North America market. The company has pioneered next-generation sequencing (NGS) platforms that are widely used in research, clinical diagnostics, and pharmaceutical development. Illumina’s innovations have significantly enhanced sequencing speed, accuracy, and affordability, making genomic analysis more accessible across academic, healthcare, and biotech sectors.
Thermo Fisher Scientific Inc.
Thermo Fisher Scientific plays a crucial role in advancing DNA sequencing through its comprehensive portfolio of sequencing instruments, reagents, and bioinformatics tools. The company supports a wide range of applications, including oncology, infectious disease monitoring, and drug discovery. Thermo Fisher’s strategic acquisitions and partnerships have strengthened its capabilities, enabling seamless integration of sequencing into clinical and industrial workflows across North America.
Pacific Biosciences (a part of QIAGEN)
Pacific Biosciences, now under QIAGEN, is recognized for its long-read sequencing technology, which enables high-resolution genome mapping and structural variant detection. This innovation addresses limitations of short-read sequencing and is increasingly adopted in complex genomics research, rare disease studies, and precision medicine. PacBio’s contributions have expanded the scope of DNA sequencing applications, particularly in resolving challenging genomic regions critical for scientific discovery.
TOP STRATEGIES USED BY KEY MARKET PARTICIPANTS
Expanding Product Portfolios through Innovation and Acquisitions
Leading companies are continuously enhancing their offerings by developing advanced sequencing platforms, consumables, and integrated software solutions. Strategic acquisitions also play a vital role in expanding technological capabilities and diversifying application reach within the DNA sequencing ecosystem.
Strengthening Clinical and Research Collaborations
Major players are forming partnerships with academic institutions, hospitals, and pharmaceutical firms to integrate sequencing into clinical practice and therapeutic development. These collaborations help accelerate the translation of genomic insights into actionable medical applications, reinforcing market leadership.
Investing in AI-Driven Genomic Data Analysis Tools
With the exponential growth of genomic data, companies are investing heavily in artificial intelligence and machine learning-based analytics to improve interpretation speed and accuracy. These tools enhance utility for clinicians and researchers, strengthening the value proposition of sequencing services in North America.
COMPETITIVE LANDSCAPE
The North America DNA sequencing market is highly competitive, characterized by the presence of established global leaders and emerging regional innovators. Companies such as Illumina, Thermo Fisher Scientific, and Pacific Biosciences dominate due to their cutting-edge technologies, extensive product portfolios, and strong distribution networks. These firms continually invest in R&D to refine sequencing accuracy, reduce costs, and expand usability in both research and clinical settings.
At the same time, mid-sized companies and startups are carving out niche positions by focusing on specialized applications such as single-cell sequencing, epigenetics, and portable sequencing devices. Startups like Oxford Nanopore Technologies and Element Biosciences are gaining traction with novel platforms designed for decentralized use and real-time analysis.
Competition is further intensified by strategic moves such as mergers, acquisitions, and collaborative agreements aimed at accelerating product development and enhancing market access. Regulatory support and growing investment in genomic medicine are driving industry consolidation while encouraging new entrants to challenge traditional business models.
As demand for personalized medicine and large-scale population genomics expands, competition is shifting toward integrated solutions that combine hardware, consumables, and advanced bioinformatics. Companies that can deliver comprehensive, scalable, and user-friendly sequencing ecosystems are best positioned to lead the evolving market landscape in North America.
RECENT MARKET DEVELOPMENTS
- In February 2024, Illumina launched a new high-throughput sequencing platform designed specifically for clinical laboratories, aiming to improve diagnostic turnaround times and scalability for hospitals and reference labs across North America.
- In June 2024, Thermo Fisher Scientific announced a strategic partnership with a leading bioinformatics firm to integrate AI-driven genomic data analysis into its sequencing workflow, enhancing interpretation accuracy and supporting clinical decision-making.
- In October 2023, Pacific Biosciences introduced an updated version of its long-read sequencing system optimized for structural variant detection, targeting research institutions and biopharma clients seeking higher resolution in complex genomic regions.
- In March 2024, a coalition of North American sequencing service providers formed a collaborative network to standardize genomic data formats, improve interoperability with electronic health records, and streamline regulatory compliance for clinical applications.
- In November 2024, a U.S.-based genomics startup secured significant venture capital funding to scale its operations in decentralized sequencing, focusing on point-of-care diagnostics and mobile health applications tailored for rural and underserved communities in North America.
MARKET SEGMENTATION
This research report on the North America DNA sequencing market is segmented and sub-segmented into the following categories.
By Product Type
- Instruments
- Consumables
- Other Product Types
By Sequencing Type
- Sanger Sequencing
- Next-generation Sequencing
- Other Sequencing Types (Third Generation Sequencing, Clinical Sequencing)
By Application
- Diagnostics
- Personalized Medicine
- Other Applications (Reproductive Health, Consumer Genomics)
By End User
- Hospitals and Healthcare Organizations
- Academics and Research Institutions
- Pharmaceutical and Biotechnology Companies
By Country
- United States
- Canada
- Mexico
- Rest of North America
Frequently Asked Questions
1. What is the projected growth of the North America DNA sequencing market?
The North America DNA sequencing market is expected to grow at a CAGR of 12.2% from 2025 to 2033.
2. What are the key drivers of the North America DNA sequencing market?
The North America DNA sequencing market is driven by advancements in precision medicine, increasing applications in disease diagnosis, and declining sequencing costs.
3. What challenges does the North America DNA sequencing market face?
The North America DNA sequencing market faces challenges such as high costs, ethical concerns, and data security issues.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
