Europe Lateral Flow Assay Market Size, Share, Trends & Growth Forecast Report By Product, Application, Technique, End User and Country (UK, France, Spain, Germany, Italy, Russia, Sweden, Denmark, Switzerland, Netherlands, Turkey, Czech Republic and Rest of Europe) - Industry Analysis, From (2025 to 2033)
Europe Lateral Flow Assay Market Size
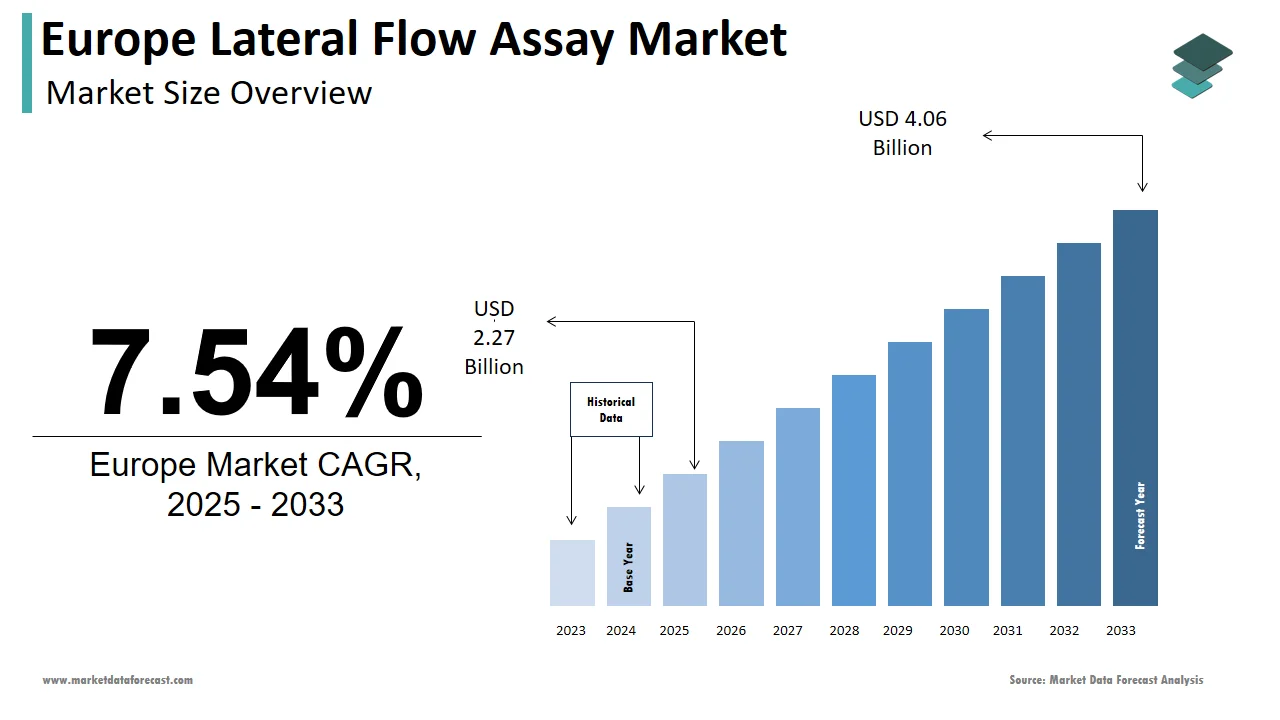
The lateral flow assay market size in Europe was valued at USD 2.11 billion in 2024. The European market is estimated to be worth USD 4.06 billion by 2033 from USD 2.27 billion in 2025, growing at a CAGR of 7.54% from 2025 to 2033.
MARKET DRIVERS
Rising Frequency of Infectious Diseases
Lateral flow assays (LFAs) are the technology behind low-cost, simple, rapid, and portable detection devices popular in biomedicine, agriculture, food, and environmental sciences. Lately, these types of assays have attracted considerable attention due to their potential to provide an immediate diagnosis to patients. The variety and interpretation of these results and parameters are used for the assessment of the assay.
The European lateral flow assay market is being propelled ahead by the rising frequency of infectious diseases, the rapidly aging population, the growing demand for point-of-care testing, and home-based lateral flow assay equipment.
Growing Preference for Point-of-Care Devices
In addition, increasing point-of-care testing devices as they are simple and portable. In addition, they produce faster results, resulting in better patient outcomes. Growing acceptance of smart devices, increased use of the lateral flow assay kits, and benefits of LFA-based fast tests over laboratory testing all contribute to the overall growth of the Europe lateral flow assay market. Since the need for rapid diagnosis of the coronavirus has been a priority since the pandemic began, it has had a beneficial impact on the lateral flow tests industry.
Technological advancements in the field of lateral flow tests have also benefited the industry. In recent years, significant advances in LFA development have included new signal amplification techniques, the introduction of new labels, improved quantification systems, and simultaneous detection.
Increased Government Participation in Infectious Disease Control
Increased government participation in controlling infectious spread, diagnosis, and prevention, and the launch of a diagnosis system in-home care settings are expected to contribute to the growth of European lateral flow assay market during the forecast period. Despite the fact that non-communicable diseases are the leading cause of morbidity and mortality, infectious diseases continue to be a major public health concern. This factor will be a significant driver of the Europe lateral flow assay market in the coming years.
MARKET RESTRAINTS
Consumer Reluctance to Shift from Traditional Diagnostic Methods
Consumer reluctance to convert from old diagnostic procedures to modern methods and the imposition of significant excise duty on medical equipment trading are anticipated to limit the market expansion. In addition, due to reimbursement issues, lateral flow assay-based diagnostics have had limited popularity in this region.
COUNTRY LEVEL ANALYSIS
Geographically, the Europe lateral flow assay market accounted for the second-largest proportion owing to the growing demand for decentralized laboratory tests and is continued to be an important market for lateral flow assay tests. Furthermore, the presence of a well-developed healthcare system boosts this market tremendously. The market is predicted to grow fast because of the increased incidence of HIV/AIDS and other infectious diseases in Europe. According to the European Centre for Disease Prevention and Control, nearly 2.5 million individuals in Europe had infected with HIV in 2020. The COVID-19 cases have been recorded in the European countries, including the United Kingdom, Spain, Germany, Italy, and France, and mass testing could be one strategy to combat the pandemic. As a result of the pandemic, numerous government agencies are focusing on mass COVID-19 testing and implementing a number of measures to increase the rate of testing in their respective countries.
Increased use of modern infrastructure, the number of diagnostic facilities, and public awareness are all likely to boost the market in the area, contributing to the region’s extraordinary share of the global market revenue throughout the forecast period. In addition, the pandemic had a significant impact on the region, compelling the government to conduct mass screening programs and promote the growth of Europe lateral flow assay market.
Germany accounted for the largest share of the European lateral flow assay market, and it is anticipated to register a significant share during the forecast period. The market is likely to be driven by an increase in the prevalence of respiratory disorders and increased investments in the development of new diagnostics solutions, and the presence of well-established healthcare infrastructure.
KEY MARKET PARTICIPANTS
A few of the notable companies operating in the Europe Lateral Flow Assay Market profiled in this report are Alere Inc., F. Hoffmann-La Roche AG, Danaher Corporation, Siemens AG, Becton, Dickinson and Company, bioMérieux SA, Johnson & Johnson, Bio-Rad Laboratories Inc., Thermo Fisher Scientific Inc., QIAGEN N.V., and PerkinElmer Inc.
MARKET SEGMENTATION
This Europe lateral flow assay market research report is segmented and sub-segmented into the following categories.
By Product
-
Reader
-
Kit
By Application
-
Clinical
-
Veterinary
-
Drug Development
-
Food Safety
By Technique
-
Competitive
-
Multiplex
-
Sandwich
By End User
-
Hospitals and Clinics
-
Home Care
-
Diagnostic Laboratories
-
Pharmaceutical and Biotechnology Companies
By Country
- UK
- France
- Spain
- Germany
- Italy
- Russia
- Sweden
- Denmark
- Switzerland
- Netherlands
- Turkey
- Czech Republic
- Rest of Europe
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2000
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com