Global Orphan Drugs Market Size, Share, Trends & Growth Analysis Report – Segmented By Type (Biological Orphan Drugs & Non-Biological Orphan Drugs), Therapeutic Type and Region (North America, Europe, Asia-Pacific, Latin America, Middle East and Africa) - Industry Forecast (2024 to 2032)
Global Orphan Drugs Market Size
The global orphan drugs market size was valued at USD 166.35 billion in 2024. As per our report, the size of the market is estimated to be worth USD 328.68 billion by 2033 and USD 179.43 billion in 2025, growing at a CAGR of 7.86% during the forecast period.
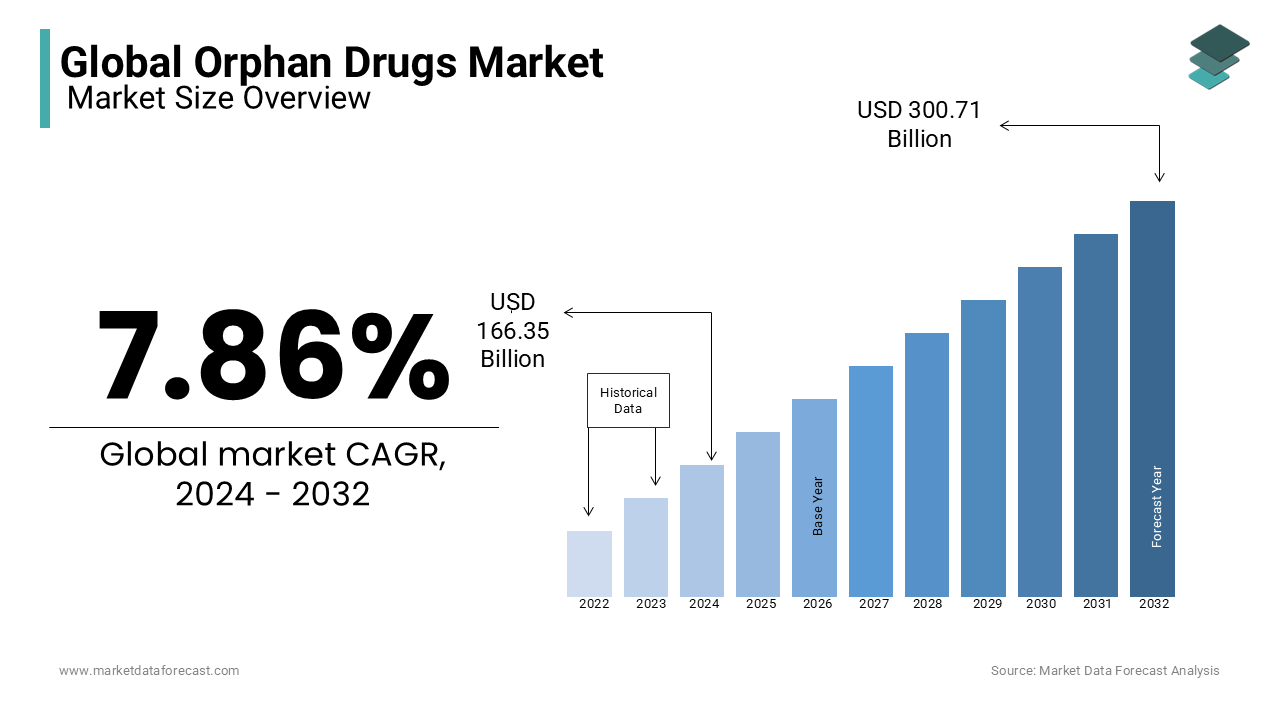
Current Scenario of the Global Orphan Drugs Market
According to an FDA report, it has approved more than 600 drugs since the Orphan Drug Act in 1983. Among those, 14% of orphan drugs are accepted with additional non-orphan indications. The ODA Act has gained acceptance from many countries and boosted the manufacturing of orphan drugs to treat related diseases. This ODA act had come up with financial and non-financial considerations to assure availability and access to orphan drugs. Under this act, government agencies are offering tax credits and subsidies favoring the patient and pharmaceutical companies, which are positively impacting the market.
MARKET DRIVERS
The growing prevalence of rare diseases is majorly propelling the growth of the orphan drugs market.
Over 30 million Americans are recognized with rare diseases, whereas 200,000 people have chronic or acute conditions such as cancer, autoimmune disorders, and digestive disorders, among others. In the U.S., biopharmaceutical companies focus on introducing innovative vaccines or drugs for rare diseases as there are no treatment options for some unique conditions. There are approximately 7000 rare diseases, and only five have specific treatments. Advancements in studies of genomics will help researchers to develop promising medicines. Studies found that 80% of the origin of the rare disease is genetic. In 2020, COVID-19 disease became a unique, challenging factor for researchers to determine effective treatment procedures.
Government incentives and favorable regulations by the regulatory bodies for orphan drugs boost the market’s growth rate.
In 1983, the U.S. Congress passed The Orphan Drug Act, which offers incentives to pharmaceutical companies that manufacture orphan drugs in the form of tax credits, grants, and exclusive marketing rights for orphan drugs to encourage pharmaceutical companies to invest in R&D activities for rare diseases. The governments further support the pharmaceutical companies by facilitating favorable regulations for the development and approval of orphan drugs. Furthermore, the governments have been providing funding for the R&D of orphan drugs and support for patient access to these drugs through reimbursement policies and insurance coverage.
The growing demand for orphan drugs, increasing awareness among people and healthcare professionals regarding the benefits of orphan drugs, and rising demand for quality healthcare are contributing to the growth of the global market. Factors such as the growing patient advocacy and support, increasing number of advancements in genetic research and personalized medicine, growing investments for the R&D of orphan drugs by private organizations, an increasing number of collaborations and partnerships among pharmaceutical companies, rising aging population and increasing prevalence of age-related diseases accelerates the market’s growth rate. Furthermore, the growing adoption of technological advancements in drug development, rising awareness and education about rare diseases among people, increasing demand for effective treatments for rare diseases and high unmet medical needs in the rare disease patient population fuel the growth rate of the orphan drugs market.
MARKET RESTRAINTS
High costs associated with the R&D of orphan drugs majorly hamper the market growth.
In addition, factors such as limited patient population, poor awareness levels among people regarding rare diseases and orphan drugs and reimbursement issues in some countries hinder the market’s growth rate. Furthermore, competition from other therapies is another notable obstacle to the orphan drugs market.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024 to 2032 |
|
Segments Covered |
By Type, Therapeutic, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis; DROC, PESTLE Analysis, Porter's Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leader Profiled |
Johnson and Johnson, Novartis, Celgene, and AbbVie are orphan drug market leaders. In addition, companies like GlaxoSmithKline, Roche, Alexion, Sanofi, Bristol Myers Squibb, Pfizer, Vertex, and Merck |
SEGMENTAL ANALYSIS
Global Orphan Drugs Market Analysis By Drug Type
The biological orphan drugs segment had the largest share of the global orphan drugs market in 2023 and is predicted to continue dominating the segment during the forecast period owing to the growing incidence of various rare diseases that are curable with biological drugs and growing investments in developing drugs for rare diseases by private organizations. The growing number of advancements in biotechnology, the favorable regulatory environment for biological orphan drugs and increasing investments for the R&D of these drugs further fuel the growth rate of the segment.
The non-biological orphan drugs segment is predicted to showcase a healthy CAGR in the coming years due to the rising prevalence of genomic studies and the rise in the number of people suffering from chronic diseases. The growing adoption of technological developments in drug discovery and development and the rising demand for personalized medicine further promote the segment’s growth rate.
Global Orphan Drugs Market Analysis By Therapeutic
The oncology segment held the largest share of the global orphan drugs market in 2023 and is predicted to grow at a healthy CAGR during the forecast period. The segmental growth is primarily driven by the growing cancer patient population worldwide and rising healthcare expenditure. As a result, companies such as Johnson & Johnson, AbbVie, and Bristol-Myers Squibb emphasize manufacturing orphan drugs related to the oncology segment.
The neurology segment is predicted to witness the highest CAGR during the forecast period owing to the growing prevalence of neurological disorders worldwide and growing support from regulatory bodies such as the Food and Drug Administration.
The hematology segment occupied a notable share of the worldwide market in 2023 and is predicted to register a healthy CAGR during the forecast period owing to the growing patient population worldwide with hematological disorders.
REGIONAL ANALYSIS
Geographically, the orphan drugs market in North America accounted for the largest share of the worldwide market in 2023 and the region's dominance is predicted to continue throughout the forecast period. The growth of the North American market is majorly driven by the growing adoption of technological advancements in drug discovery and development and the presence of notable drug manufacturers in North America. The favorable regulatory environment for orphan drugs in North America, the rising prevalence of rare diseases and the presence of sophisticated healthcare infrastructure further fuel the growth rate of the North American market. The U.S accounted for the largest share of the global orphan drugs market in 2022, as it spends nearly 20% of its GDP on health. Canada is another potential market for orphan drugs in North America and is predicted to witness a healthy CAGR during the forecast period.
The European orphan drugs market accounted for a substantial share of the global market in 2023 and is expected to grow at a promising CAGR during the forecast period. Factors such as the growing prevalence of novel drugs and the growing incidence of rare diseases across the European region boost the growth rate of the European market. In addition, the presence of numerous biotech and pharmaceutical companies and favorable regulations for the development of orphan drugs by regulatory bodies such as the European Medicines Agency (EMA) further promotes the growth of the European market. Germany, France and the UK held the most significant share of the European market in 2023.
The orphan drugs market in the Asia Pacific is estimated to hit the highest CAGR in the worldwide market during the forecast period owing to the rising prevalence of rare diseases and the presence of developing countries such as India and China. In addition, the growing awareness about rare diseases, rising need for effective treatments, increasing healthcare expenditure and the growing geriatric population in the APAC region contribute to the APAC orphan drugs market growth during the forecast period. During the forecast period, India, China, Japan and South Korea are predicted to hold the largest share of the APAC market.
The Latin American orphan drugs market held a considerable share of the global market in 2023. Factors such as the rising prevalence of rare diseases, growing demand for effective treatments and increasing focus on healthcare infrastructure development propel the regional market growth. Mexico and Brazil held the major share of the Latin American market in 2022, which is expected to repeat throughout the forecast period.
The orphan drugs market in MEA is expected to grow at a moderate CAGR during the forecast period owing to the increasing investments in healthcare infrastructure and the rising awareness about orphan drugs.
KEY PLAYERS IN THE GLOBAL ORPHAN DRUGS MARKET
Johnson and Johnson, Novartis, Celgene, and AbbVie are orphan drug market leaders. In addition, companies like GlaxoSmithKline, Roche, Alexion, Sanofi, Bristol Myers Squibb, Pfizer, Vertex, and Merck are also playing a promising role in the orphan drugs market.
RECENT HAPPENINGS IN THIS MARKET
- In March 2023, Cyclerion Therapeutics, Inc. announced that Zagociguat received the orphan drug status from the USFDA. Zagociguat is used in the treatment procedures of mitochondrial disease.
- In February 2023, Asklepios BioPharmaceutical, Inc. announced that AB-1003 had gained the Orphan Drug Designation by the European Commission. AB-1003 is now an orphan drug and is used in the treatment procedures of limb-girdle muscular dystrophy (LGMD).
- In February 2019, Roche bought Spark Therapeutics. This deal helped Roche to make its presence more robust in advanced therapies. On the other end, Spark Therapeutics was named the first company in the United States to receive gene therapy approval for their Luxturna. Luxturna is a therapy to treat rare inherited eye diseases.
- In January 2019, an agreement between Medunik Canada and HRA Pharma had legal rights for marketing and distributing an orphan drug named Lysodren, which helps treat inoperable adrenal cortical carcinomas.
- In April 2018, AveXis was purchased by Novartis. AveXis is a Biotechnology Company based out of the United States that develops therapies for rare disorders related to neurological genetics. AVXS-101 is the lead candidate of AveXis, is a gene-replacement therapy to treat a rare disease called SMA (spinal muscular atrophy)
- In December 2017, Aprecia Pharmaceuticals collaborated with Cycle Pharmaceuticals to introduce 3D-printed orphan drugs in foreseen years.
- In November 2016, AbbVie, one of the leaders in manufacturing orphan drugs, received the Orphan Drug Designation from the FDA.
DETAILED SEGMENTATION OF THE GLOBAL ORPHAN DRUGS MARKET INCLUDED IN THIS REPORT
This research report on the global orphan drugs market has been segmented & sub-segmented into the following categories.
By Drug Type
- Biological
- Non-Biological
By Therapeutic
- Hematology
- Neurology
- Oncology
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
Which region is growing the fastest in the global orphan drugs market?
The Asia-pacific region is expected to grow the fastest in the global market during the forecast period.
Does this report include the impact of COVID-19 on the orphan drugs market?
Yes, we have studied and included the COVID-19 impact on the global orphan drugs market in this report.
Which are the major players operating in the orphan drugs market?
Johnson and Johnson, Novartis, Celgene, AbbVie, GlaxoSmithKline, Roche, Alexion, Sanofi, Bristol Myers Squibb, Pfizer, Vertex, and Merck are some of the major companies operating in the global orphan drugs market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
