Global Preventive Vaccines Market Size, Share, Trends & Growth Forecast Report - Segmented By Vaccine Type (Live, Attenuated Vaccines, Inactivated Vaccines, Toxoid Vaccines, Subunit Vaccines, Conjugate Vaccines, DNA Vaccines, and Recombinant Vector Vaccines), End User and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) - Industry Analysis (2024 to 2032)
Global Preventive Vaccines Market Size (2024 to 2032)
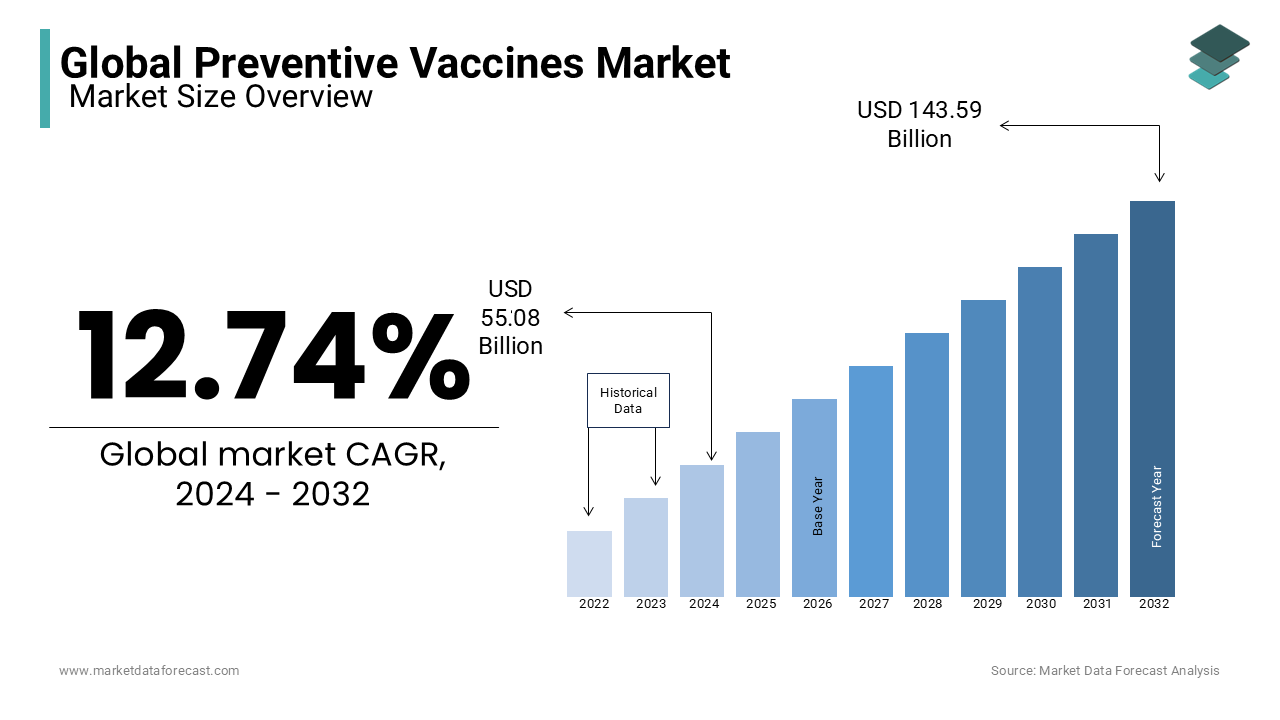
In 2023, the global Preventive Vaccines Market was valued at USD 48.86 billion and it is expected to reach USD 143.59 billion by 2032 from USD 55.08 billion in 2024, growing at a CAGR of 12.74 % during the forecast period.
Preventive vaccines are created to kill the bacteria or viruses in our body through antibodies produced by the prevention vaccination. The presence of antibodies in the body reduces the chance of getting affected by any virus. Although vaccines were solely responsible for the global eradication of small polio, several vaccine-preventable diseases, such as measles and mumps, are still a threat. COVID-19 has been recently added to the list. The vaccine market accounts for only 5% of the global pharmaceuticals market. However, recently there has been substantial growth owing to the rising incidences of cancer & HIV cases. Nevertheless, there has been rapid growth over recent years, with the vaccine market magnifying in value from USD 5 billion in 2000 to almost USD 25 billion in 2013. Furthermore, World Health Organization (WHO) growth estimations indicate the market may increase in value to nearly 60 billion in 2020, with the arrival of new therapeutic, preventive, and adult vaccines.
Current Scenario of the Global Preventive Vaccines Market
The global preventive vaccines market is different from any other product market. Compared to the pharmaceutical market, it is relatively small, although proliferating. There are presently nearly 120 new vaccines in the pipeline of various multinational companies across the globe set to hit the market in the next few years. The global Preventive Vaccines Market concentrates on both the supply and demand sides. It is highly regulated and primarily dependent on public purchasers and donor policies. It has distinct features that increase the complexity of assessing and understanding pricing and procurement in their context.
MARKET DRIVERS
The growing infectious disease burden significantly contributes to the global preventive vaccines market growth.
Infectious diseases such as polio, measles, pneumonia, HIV, and other diseases can be prevented by vaccination. The concept of vaccination has dramatically reduced the mortality, morbidity, and complications associated with a wide range of infectious diseases. More than 1400 clinical trials evaluating various types of preventive vaccines have been registered, indicating the rapid pace of development in this field. Over 1.5 million children under 5 love their lives to vaccine-preventable diseases yearly. By WHO, more than 1400 epidemic events were tracked in 172 countries between 2011 and 2018. Approximately 3 million deaths are preventable with effective immunizations every year.
Growing investments by the key market players and increasing government funding for vaccine developments propel global market growth. Furthermore, the rising prevalence of the disease, technological advancements to decrease the new vaccine development time, and initiatives by NGOs contribute generously to the growth of preventive vaccines.
MARKET RESTRAINTS
However, massive capital expenditures, stringent regulatory policies, and high costs associated with treatment are a few factors inhibiting the growth of the global Preventive Vaccines Market. Furthermore, the lack of skilled and efficient labor required for manufacturing the vaccine among the pharmaceutical and biotechnology companies is curbing the market growth rate. In addition, the high cost of the raw materials required in the laboratories to develop a vaccine impedes the market’s growth rate.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 to 2032 |
|
Base Year |
2023 |
|
Forecast Period |
2024 to 2032 |
|
Segments Covered |
By Product Type, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter's Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Market Leaders Profiled |
GlaxoSmithKline PLC, Merck and Company, Bavarian Nordic, CSL Limited, Emergent BioSolutions Inc., Novartis AG, Johnson and Johnson, Medimmune LLC, Pfizer |
SEGMENTAL ANALYSIS
Global Preventive Vaccines Market Analysis By Vaccine Type
Over 20% of the market share was occupied by the live, attenuated vaccines segment in the global preventive vaccines market in 2023. Live attenuated vaccine helps to prevent natural infections that an individual experiences in their lifetime. Live-attenuated vaccines are beneficial for diseases such as influenza, chickenpox, measles, polio, and TB. In addition, it produces an immune system to fight against diseases supporting segment growth.
The recombinant vector and conjugate vaccines segments were in second and third position in leading the market share. An increasing number of people with cancer and other Infectious diseases significantly influence this segment's growth. In addition, increasing expenditure on healthcare, especially in urban areas, is promoting the segment's growth.
The inactivated vaccines segment has been growing faster for the past few years. The rise in awareness among physicians over the availability of different vaccines for various diseases is gearing up the market's growth rate. Inactivated vaccines are made of dead or inactivated viruses and bacteria, which is different from other diseases.
Global Preventive Vaccines Market Analysis By Application
The pneumococcal segment held the dominating share of the preventive vaccines market in 2023 with the rise in pharmaceutical companies’ investments by private and public organizations. According to the statistics published by World Health Organization (WHO), nearly 15% of deaths in children below five years of age were due to pneumonia in 2019. Some viruses, bacteria, etc can cause pneumonia. Therefore, the neglect of vaccines results in an increased mortality rate. However, the growing prevalence of improving treatment procedures to promote the patient's well-being will eventually outshine the market growth.
The Influenzas segment also has significant growth in the market. According to Families Fighting Flu (FFF), nearly 1 billion people worldwide suffer from influenza, which is affected by seasonal changes yearly. The burden of flu disease is increasing rapidly due the factors such as increasing virus spread, seasonal changes, and environmental pollution. World health organization tracks the spread of the influenza virus and how the vaccine works for the affected people. As a result, researchers are focusing on developing the vaccine in advanced stages based on the patient's behavior.
REGIONAL ANALYSIS
North America accounted for the most significant share of the global market in 2023, owing to the rapid adoption of the latest technologies and innovative drugs & vaccines. In addition, the rise in pharmaceutical companies’ scale and ongoing research in the biotechnology field is leveraging the growth of the preventive vaccines market in North America. In Canada, the National Advisory Committee on Immunization plays a major role in recommending newly approved vaccines to public health experts. The Canada Immunization Guide (CIG) depends on the recommendations of the national advisory committee and other factors. The committee also focuses on conducting programs for vaccine dosages and administration by the people to prevent the spread of deadly diseases. During the pandemic, Canada had the highest market share due to the government's strict rules of getting vaccinated to prevent the spread of the disease. According to previous studies, it is proven that childhood vaccination is the most effective in controlling and preventing disease. Therefore, the Healthcare sector in America took registrations of newborn babies and administered the vaccine at different levels to reduce the spread of dangerous diseases in their life.
Europe had a substantial share of the global market in 2023 due to the rise in the geriatric population and growing economies in this region. To reduce the cases of chicken pox and polio, the German government follows strict vaccination measures to decrease the mortality rate. In addition, the government has started investing in making advanced drugs for chicken pox and polio to see vaccine-positive outcomes in the region.
Asia-Pacific is forecasted to have a significant CAGR in the coming years, increasing support from the government by launching reimbursement schemes in favor of ordinary people. In recent years, China has held the maximum share in the market growth due to the increasing use of preventive vaccines due to the development of different types of diseases in their country. This prevention vaccine helps produce antibodies in the body to fight against viruses and reduce mortality.
Latin America is expected to grow at a steady CAGR during the forecast period. Emerging economies such as Mexico and Brazil contribute significantly to regional market growth.
The market in the MEA had a small portion of the global market in 2023. However, the market is gaining attention due to the growing awareness regarding preventive vaccines and is expected to grow at a considerable CAGR during the forecast period.
KEY PARTICIPANTS IN THE GLOBAL PREVENTIVE VACCINES MARKET
Some of the promising companies dominating the global preventive vaccines market profiled in the report are GlaxoSmithKline PLC, Merck and Company, Bavarian Nordic, CSL Limited, Emergent BioSolutions Inc., Novartis AG, Johnson and Johnson, Medimmune LLC, Pfizer, Inc., and Sanofi Pasteur.
Vaccine developers are making significant efforts to ensure their candidates are clinically and commercially competent to achieve a competitive edge. As a result, several companies have received substantial funding for their proprietary product portfolios; majorly, they are mid-sized companies based in North America. In addition, around 70 companies in different regions worldwide claim to provide contact development, fill/finish, and regulatory support, besides manufacturing services.
RECENT HAPPENINGS IN THIS MARKET
- In 2022, Pfizer Inc. and BioNTech SE partnered and announced that the Pfizer-BioNTech COVID-19 vaccine got approval from the U.S. Food and Drug Administration, which can be given to children six months through 4 years of age for the prevention of Covid-19.
- In 2021, Merck and Sanofi developed a vaccine to prevent diphtheria, tetanus, pertussis, poliomyelitis, and hepatitis B for children and adolescents. This six-in-one vaccine is given to people below five years of age and is available in the US.
- In February 2020, GSK and CEPI recently announced that they would collaborate to develop a vaccine for the nCoV virus with the help of vaccine adjuvant platform technology developed by GSK. As a result, a more significant number of people are benefitting from the antigen-sparing effect. The primary goal of the collaboration is to develop vaccines rapidly by decreasing the dose of vaccine antigens to provide a cure for a large group of people affected by the coronavirus.
- In January 2020, Serum Institute of India Pvt. Ltd and Staten's Serum Institute have entered into a strategic partnership to intensify vaccine development for tuberculosis. Serum Institute has developed a 10-valent pneumococcal conjugate vaccine called pneumonia, which contains CRM197, which the institute also produces under Pfenex expression technology. However, the World Health Organization has prequalified the vaccine.
DETAILED SEGMENTATION OF THE GLOBAL PREVENTIVE VACCINES MARKET IN THIS REPORT
This research report on the global preventive vaccines market has been segmented and sub-segmented based on the vaccine type, application, and region.
By Vaccine Type
- Live, Attenuated Vaccines
- Inactivated Vaccines
- Toxoid Vaccines
- Subunit Vaccines
- Conjugate Vaccines
- DNA Vaccines
- Recombinant Vector Vaccines
By End-User
- Pediatric Vaccine
- Pneumococcal
- MMR
- Varicella
- Hepatitis
- Poliovirus
- HIB
- Others
- Adult Vaccines
- Influenza
- Cervical Cancer
- Hepatitis
- Zoster
- Others
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
How much was the global preventive vaccines market worth in 2023?
The global preventive vaccines market size was worth USD 48.86 billion in 2023.
Which segment by vaccine type had the major share of the preventive vaccines market in 2023?
Based on the vaccine type, the live, Attenuated vaccines led the market in 2023.
Which region accounted for the largest share in the global preventive vaccines market in 2023?
Geographically, the North American had the largest share of the global market in 2023.
What are some of the major players in the preventive vaccines market?
GlaxoSmithKline PLC, Merck and Company, Bavarian Nordic, CSL Limited, Emergent BioSolutions Inc., Novartis AG, Johnson and Johnson, MedImmune LLC, Pfizer, Inc., and Sanofi Pasteur are some of the noteworthy players in the global preventive vaccines market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from $ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: [email protected]
