Global Non-Invasive Prenatal Testing (NIPT) Market Size, Share, Trends & Growth Forecast Report – Segmented By Instruments (Ultrasound, NGS, PCR and Microarray), Method (FCMB and Cf-DNA), Application (Trisomy, Microdeletion, Genetics and Rh factor) and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) - Industry Analysis (2025 to 2033)
Global Non-Invasive Prenatal Testing Market Size
The global NIPT market size was valued at USD 3.01 bn in 2024. The global non-invasive prenatal testing market is further estimated to grow at a CAGR of 15.6% during the forecast period and be worth USD 11.10 billion by 2033 from USD 3.48 billion in 2025.
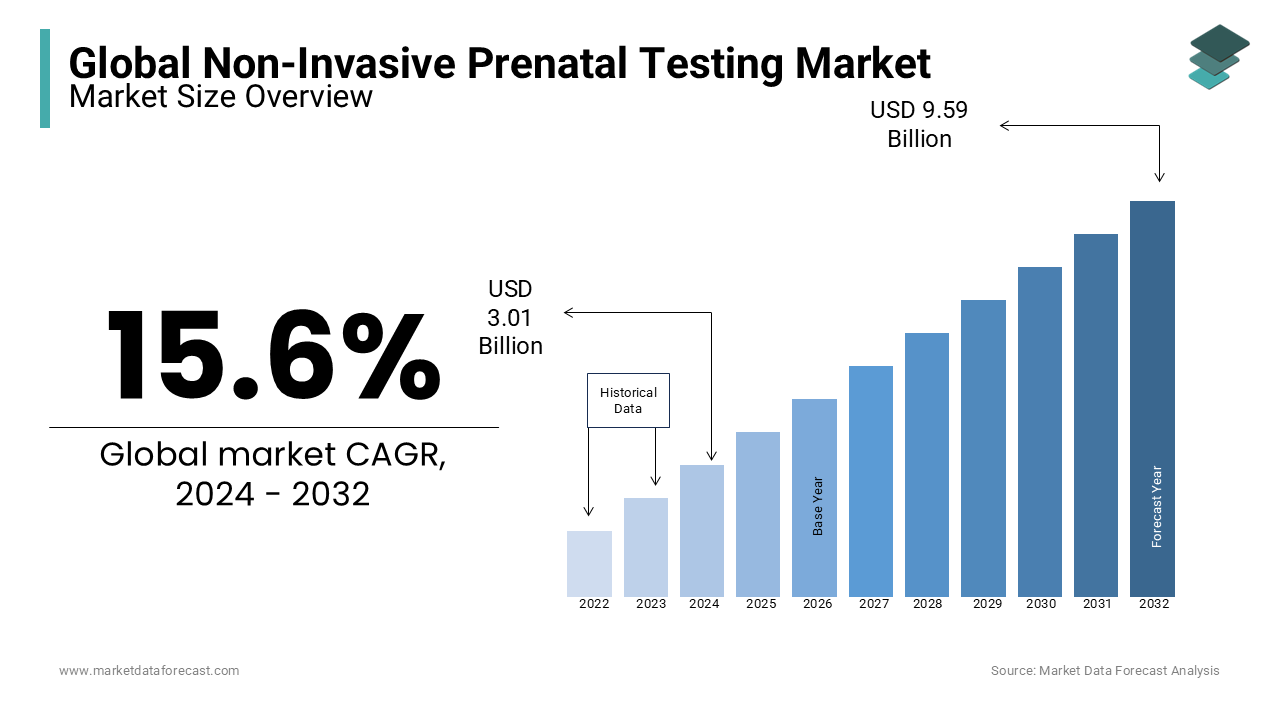
Non-invasive prenatal testing (NIPT) is a famous prenatal screening test used to evaluate fetal chromosomal abnormalities, such as trisomy 21, trisomy 18, and trisomy 13. The success rate of NIPT is high, due to which the adoption of NIPT is increasing worldwide. NIPT believed as the safest choice for pregnant women as it just takes only a tiny blood sample for the procedures, unlike invasive surgeries. With the help of NIPT, parents can detect chromosomal abnormalities in the early stages of pregnancy and take the required actions.
MARKET DRIVERS
The growing number of pregnancies worldwide is one of the major factors propelling the non-invasive prenatal testing market growth.
Women of the present generation are showing interest in having children at a later age. The preference among pregnant women towards non-invasive prenatal testing increases as this technique is safe, non-invasive, and gives high-accuracy results. To conduct non-invasive prenatal testing, a small blood sample would be taken from the pregnant woman's fetus and tested to evaluate the presence of chromosomal abnormalities. The number of pregnant cases grows worldwide, resulting in a growing number of NIPT procedures. People who get pregnant at a later age or with a history of genetic diseases are likely to have a high risk for fetal chromosomal abnormalities.
The rising support from the governments of several countries in favor of NIPT further fuels the NIPT market growth.
The governments of some countries discussed with the insurance companies the possibility of covering the costs of the NIPT under the insurance policies and recommended NIPT as a first-line screening test for chromosomal abnormalities. The governments of some countries have been offering to fund the R&D of NIPT and have taken several initiatives in favor of NIPT procedures. The favorable reimbursement policies promote the NIPT market growth. The procedures of NIPT have favorable reimbursement policies in the U.S., Canada, the UK, France, Italy, Spain, Germany, Japan, South Korea and others.
The growing awareness among healthcare professionals and the public regarding the advantages of NIPT boosts the growth rate of the non-invasive prenatal testing market.
The growing number of initiatives by governmental organizations and patient advocacy groups to promote awareness among people regarding the benefits of NIPT and the high costs associated with invasive procedures fuel the NIPT market growth. NIPT procedures are less risky and less invasive than traditional testing procedures, and the affordability is higher than traditional testing methods. The growing adoption of technological advancements to enhance the efficacy of the NIPT and an increasing number of NIPT innovations support the market's growth rate.
Furthermore, the greater accuracy and sensitivity of NIPT compared to traditional prenatal testing methods, rising demand for early detection and diagnosis of fetal abnormalities to ensure proper medical care for the baby and the mother, increasing incidence of chromosomal disorders, such as Down syndrome, Turner syndrome, and Edwards syndrome and advancements in genomic technologies, such as next-generation sequencing (NGS) and microarray analysis promote the NIPT market growth. The growing adoption of cell-free DNA (cfDNA) analysis for NIPT allows for the detection of fetal chromosomal abnormalities using maternal blood samples, rising demand for personalized medicine and precision diagnostics, increasing healthcare expenditure and government initiatives to improve prenatal care and reducing infant mortality rates and rising availability of NIPT in emerging markets, such as China and India further support the growth rate of the NIPT market.
MARKET RESTRAINTS
Poor awareness levels among healthcare providers and patients regarding the advantages associated with NIPT are significant factors hampering the NIPT market growth.
In addition, factors such as the high costs of NIPT, limited availability of NIPT in some of the developing countries, the presence of stringent regulations in some of the countries, and ethical concerns associated with the usage of NIPT in some of the countries are inhibiting the growth of the global non-invasive prenatal testing market. Furthermore, the availability of other prenatal screening and diagnostic tests hinders the market growth.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Instruments, Methods, Applications, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter’s Five Forces Analysis; Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key Market Players |
G.E. Healthcare (U.S.), Koninklijke Philips N.V. (Netherlands), Illumina, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Pacific Biosciences of California, Inc. |
SEGMENTAL ANALYSIS
By Instruments Insights
The ultrasound segment had a leading share of the global non-invasive prenatal testing market in 2023 and the segment's domination during the forecast period. Ultrasound-based NIPT is one of the safest and most reliable to figure out the chromosomal abnormalities in a developing fetus. Therefore, the awareness of the benefits of ultrasound-based NIPT is majorly driving segmental growth. In addition, ultrasound is cost-effective compared to the other invasive treatment methods, which is anticipated to promote the segment's growth rate. Furthermore, the growing demand for prenatal screening and increasing adoption of technological developments to enhance the quality of ultrasound-based NIPT are expected to boost segmental growth.
The NGS segment held a considerable share of the global market in 2022 and is expected to grow at a healthy CAGR during the forecast period. Factors such as the ability of NGS to detect fetal abnormalities with high accuracy and specificity, the growing prevalence of genetic disorders, rising demand for early and accurate diagnosis of fetal abnormalities and advancements in NGS technology propel the segmental growth.
By Method Insights
The cf-DNA segment accounted for a significant share of the global NIPT market in 2023 and the domination of the segment is expected to continue in the foreseen years. The adoption of cf-DNA is growing significantly among healthcare professionals and patients, primarily propelling segmental growth. In addition, the high accuracy of cf-DNA is another significant attribute promoting the growth rate of the NIPT market.
The FCMB segment is anticipated to showcase rapid growth during the forecast period. The high degree of accuracy of the FCMB method in detecting fetal abnormalities including chromosomal abnormalities is one of the major factors driving segmental growth. The non-invasiveness of FCMB and growing awareness among pregnant women and healthcare providers about the benefits of NIPT further boost the growth rate of the FCMB segment.
By Application Insights
The trisomy segment captured the leading share of the global NIPT market in 2023 and the segmental domination is anticipated to continue throughout the forecast period. The growing awareness regarding trisomy disorders among healthcare professionals and increasing preference towards early detection and management support segmental growth. The rising adoption of NIPT as a first-line screening test for trisomy by pregnant women and the growing emphasis of market players on developing novel NIPT products for trisomy detection further contribute to segmental growth.
The microdeletion segment is anticipated to witness the fastest CAGR during the forecast period due to factors such as the growing prevalence of microdeletion disorders such as Prader-Willi syndrome, Angelman syndrome and Wolf-Hirschhorn syndrome and the high accuracy and reliability of NIPT in detecting microdeletions in the fetus.
By End-users Insights
The diagnostic lab's segment occupied the leading share of the NIPT market in 2023 and the domination of the segment is expected to continue during the forecast period. This is because the adoption of diagnostic labs is higher than the hospitals for NIPT. In addition, the domination of the segment can be credited to the availability of equipment and trained personnel required for NIPT among the diagnostic labs.
REGIONAL ANALYSIS
North America had the largest share of the worldwide market in 2023 and the region's dominance is expected to continue during the forecast period. The popularity of NIPT has been increasing over the last several years. It is believed to be the first-line screening test for pregnant women, one of the key factors propelling the NIPT market in North America. In 2021, the U.S. NIPT market led North America, followed by Canada. The greater awareness among healthcare professionals and patients, growing adoption of technological developments, and increasing insurance coverage propel the NIPT market in the U.S. The growing number of U.S. initiatives further promotes the growth of the U.S. NIPT market. The U.S. government took numerous initiatives to promote awareness of prenatal and genetic testing. Furthermore, the growing maternal age is boosting the NIPT market in the U.S.
Europe had the second-largest regional share in the worldwide market in 2022 and is estimated to grow at a promising share during the forecast period. Factors such as increasing awareness regarding the NIPT, the growing number of NIPT procedures, rising adoption of non-invasive methods over invasive methods such as amniocentesis and increasing incidence of miscarriages propel the regional market growth. During the forecast period, the UK, Germany, France, and Spain are anticipated to hold a significant share of the European region. In addition, the availability of new and advanced NIPT technologies among European countries promotes the growth of the European NIPT market.
Asia-Pacific is anticipated to showcase the fastest CAGR during the forecast period owing to the growing population among the APAC countries such as India and China and the increasing usage of NIPT procedures. Furthermore, the number of pregnancies is growing significantly across the APAC region, favoring the APAC NIPT market growth. In addition, the rising incidence of genetic disorders is fuelling the NIPT market in the Asia-Pacific region. Furthermore, an increasing number of initiatives from the APAC governments in favor of NIPT and the availability of funding for R&D are promoting the growth of the NIPT market in the Asia-Pacific region.
During the forecast period, Latin America is estimated to have a steady share in the global market. The rising incidence of genetic diseases is a significant factor in regional market growth.
The Middle East and African market is expected to grow at a moderate share worldwide in the coming years.
KEY MARKET PLAYERS
Some of the most promising companies holding a significant share in the Global Non-Invasive Prenatal Testing Market profiled in this report are G.E. Healthcare (U.S.), Koninklijke Philips N.V. (Netherlands), Illumina, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Pacific Biosciences of California, Inc. (U.S.), PerkinElmer, Inc. (U.S.), QIAGEN N.V. (Germany), Agilent Technologies, Inc. (U.S.) and Beijing Genomics Institute (China).
RECENT HAPPENINGS IN THIS MARKET
- In June 2019, Illumina, Inc. announced the launch of VeriSeq NIPT Solution v2, a CE-IVD NGS-based NIPT.
- In November 2018, Perkin Elmer, Inc. announced the CE-mark approval for their Vanadis NIPT system for distribution and commercialization across the European region.
- In June 2017, LifeCell partnered with LifeCodexx AG to provide rapid and affordable NIPT in India.
MARKET SEGMENTATION
This research report on the global non-invasive prenatal testing market has been segmented and sub-segmented based on the instruments, methods, applications, end-users, and region.
By Instruments
- Ultrasound
- NGS
- PCR
- Microarray
By Method
- FCMB
- Cf-DNA
By Application
- Trisomy
- Microdeletion
- Genetics
- Rh factor
By End-users
- Hospital
- Diagnostic Labs
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Frequently Asked Questions
What was the size of the non-invasive prenatal testing market worldwide in 2025?
The global non-invasive prenatal testing market size was valued at USD 3.01 billion in 2025.
Which region had the largest share in the global non-invasive prenatal testing market in 2025?
North America had the largest share in the worldwide market in 2025, followed by Europe.
Which segment by instruments led the non-invasive prenatal testing market in 2025?
Based on instruments, the ultrasound segment occupied the major share of the non-invasive prenatal testing market in 2025.
What are the companies playing a noteworthy role in the non-invasive prenatal testing market?
G.E. Healthcare (U.S.), Koninklijke Philips N.V. (Netherlands), Illumina, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Pacific Biosciences of California, Inc. (U.S.), PerkinElmer, Inc. (U.S.), QIAGEN N.V. (Germany), Agilent Technologies, Inc. (U.S.) and Beijing Genomics Institute (China) are some of the major players in the non-invasive prenatal testing market.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
