Global Point of Care Diagnostics Market (POC Testing Market) Size, Share, Trends and Growth Forecast Report – Segmented By Product (Infectious Diseases, Cardiac Markers, Oncology Markers, OTC Diagnostic Tests, Drugs Of Abuse, Blood Gas Testing, Fertility Testing, Urinalysis, Coagulation, Hematology, Glucose Monitoring, Ambulatory Chemistry and Decentralized Clinical Chemistry) and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) - Industry Analysis From 2025 to 2033
Global Point of Care Diagnostics Market Size
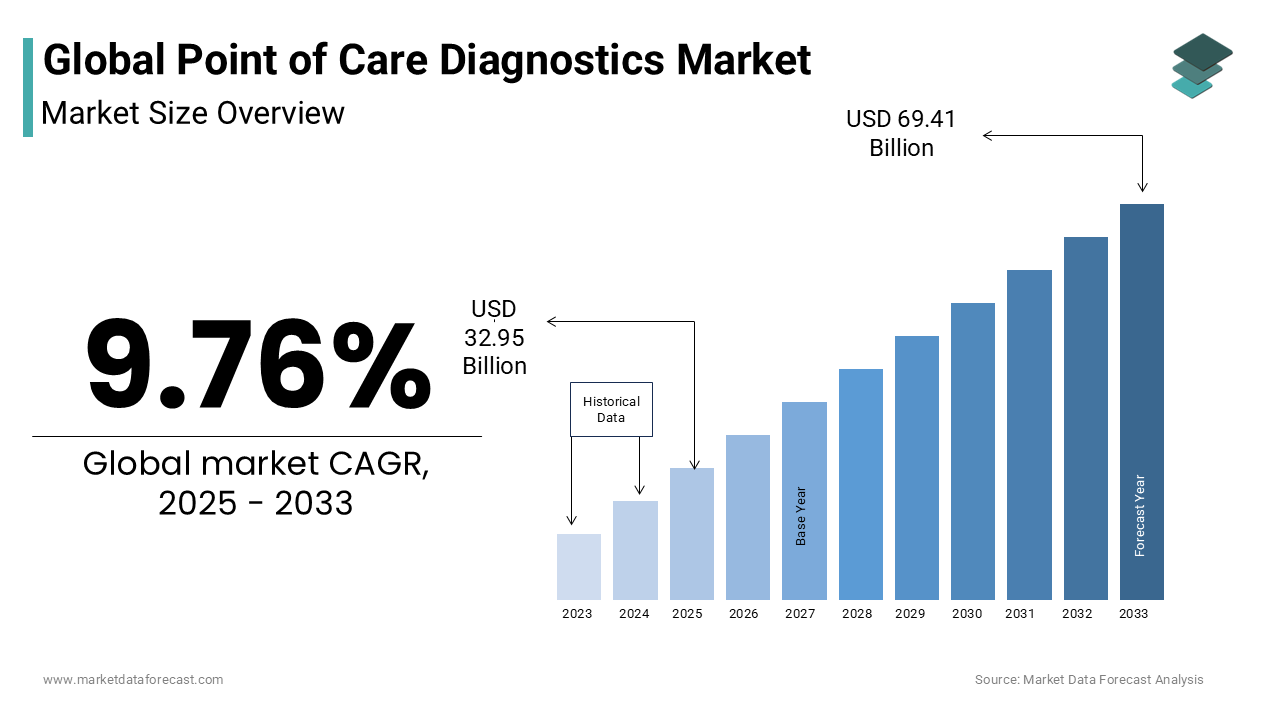
As per our report, the global point of care (POC) diagnostics market size is estimated to be growing at 9.76% CAGR during the forecast period. The global POC testing market is predicted to grow to USD 69.41 billion by 2033 from USD 32.95 billion in 2025. The point of care (POC) diagnostics market size was valued at USD 30.02 billion in 2024.
The point-of-care diagnostics (POCT) is a type of medical testing which is performed near the patient and it provides instant results. This method is to avoid sending of samples to central laboratory. The point-of-diagnostic tests can be performed by laboratory professionals, doctors or nurses, emergency first responders, and radiologists. These can be performed in hospitals, pharmacies, at home, ambulances, and paramedical support. The POCT helps to make faster decisions, and supports in improving the adherence to treatment with limited incidence of complications. The POCT includes physical tests such as pulse oximeter, blood pressure machine, and electrocardiogram and it also includes fluid tests such as blood tests, urine tests, or pregnancy tests. The point of care diagnostics market is accounted with significant growth from the past years and is anticipated to record substantial growth in the coming years. The prevalence in the chronic diseases worldwide is the significant factor contributing to the expansion of the global market.
- For Instance, according to data published by WHO, in 2022, approximately 39.0 million people are living with HIV, and around 1.3 million people acquired HIV in 2022 globally.
MARKET DRIVERS
The growing need for immediate test results and growing market penetration of EHR (Electronic Health Records) and PACS (Picture Archiving and Communication Systems) are majorly propelling the global point-of-care diagnostics market growth.
Adoption of sedentary lifestyles, unhealthy eating habits, and environmental factors are expected to surge the growth rate of the POC diagnostics market. Furthermore, the growing demand for more efficient, easy-to-use, and faster consumer devices and these portable personal care devices is fuelling the global POC testing market growth. In addition, new product launches of home-testing cancer kits at regular intervals and enhanced laboratories due to the integration of automation techniques in healthcare are estimated to boost market growth.
Increasing R&D activities to introduce new POC tests and devices for rapid disease detection and monitoring drives the progress of the point-of-care diagnostics market.
Recently in February 2018, Siemens Healthineers gained FDA approval (Food and Drug Administration) for total carbon dioxide (TCO2) and blood urea nitrogen (BUN) POC testing, increasing its market presence. The high prevalence of chronic illnesses and cancers means that a large customer base deploys cheap and instant diagnostic tests, driving the point-of-care diagnostics /testing market. It is complemented by several cheap initiatives to facilitate POC tests in diagnostic and screening procedures.
The significant increase in the chronic diseases such as diabetes, cardiovascular disorders and various infectious diseases along with growing population worldwide is creating growth opportunities for point-of-care diagnostics market. The growing population and diseases prevalence is influencing the market players to focus on development and introduction of novel products and test kits is contributing to expansion of market growth opportunities. The increased demand for the point-of-care diagnostics testing in the patient-centric healthcare owing to its rapid results in diagnosis which helps in faster treatment decisions is estimated to enhance the growth opportunities in the coming years. The escalation in the government and regulatory support initiatives is augmenting the market growth.
- For Instance, in 2022, F.Hoffmann-La Roche Ltd., was approved for the first companion diagnostic by U.S FDA for detection of patients with HER2 low metastatic breast cancer.
- For Instance, In February 2023, Anavasi Diagnostics received the FDA Emergency Use Authorization (EUA) for AscencioDx Molecular Detector and AscencioDx COVID-19 test.
MARKET RESTRAINTS
The lack of skilled people in manufacturing the kits with the latest design in some regions is a significant restraint to the global POC diagnostics market growth.
In addition, the cost is a roadblock factor, as laboratory testing tends to be less expensive than POC testing. Providers typically require the support of a laboratory to meet quality and accreditation requirements. Quality assurance can be rigorous testing performed by clinical staff rather than individuals who have been laboratory trained. All these factors are projected to hamper the market growth opportunities. The lack of accuracy is the significant factor restricting the adoption rate among the people compared to the laboratory tests is leading to restricted market growth. The high costs associated with the advanced technologies is increasing the overall costs of the kits and devices which is hindering the market growth opportunities. The limited awareness and availability in various regions especially in the underdeveloped and developing nations impedes the market expansion.
The POCT uses various different methodologies compared to the central laboratories which may implement limitations in the test results act as significant challenge to the market growth. The presence of stringent regulations for product approval and launch owing to their efficacy and safety is another major challenge for the market players due to high complexity and longer wait period. Various other challenges such as sample quality, and the usage of device, shortage of skilled professionals, insufficient documentation, challenges in connecting POCT instruments to electronic medical record are expected to impede the market growth opportunities.
REPORT COVERAGE
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 to 2033 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2033 |
|
Segments Covered |
By Product, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, Drivers, Restraints, Opportunities, Challenges; PESTLE Analysis; Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview of Investment Opportunities |
|
Regions Covered |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key Market Players |
Abbott Laboratories, Inc., Danaher Corporation, Roche Diagnostics Limited, BioMerieux, Siemens Healthcare, Beckman Coulter, Inc., Becton, Dickinson and Company, Johnson & Johnson, Alere Inc., and PTS Diagnostics. |
SEGMENTAL ANALYSIS
By Product Insights
The glucose monitoring segment led the market with a significant share in 2023 due to factors such as increasing the adoption rate of portable glucose meters with each passing year and growing awareness among diabetic patients to monitor glucose levels in the blood at regular intervals.
The cancer markers market segment is to grow at a high pace. The oncology makers segment is another lucrative segment among all and is anticipated to register a healthy CAGR during the forecast period owing to an increase in cancer cases and increasing death of cancer due to the delay in the identification of cancer in the body. Early detection of tumors can provide a more effective treatment for the tumor patient. Point-of-care (POC) testing is used for the patient to get the result in a short period with less cost. However, these patients must need further diagnosis if the tumor is detected in this POC testing by increasing advancement in using biomarkers in POC devices to know cancer in the patient more accurately.
The cardiac maker segment is also expected to perform well during the global POPC testing market forecast period during the foreseen years. Hospitals are increasingly using rapid results for cardiac-based devices to measure cardiac biomarkers. Some groups, such as the U.K. National Institute for Health and Care Excellence and the European Society of Cardiology, have accepted similar decision-making protocols for rapidly measuring cardiac troponin. Patients suffering from chest pain undergo these tests to know the quick result of their heart condition.
By End-User Insights
The clinic segment dominated the market, contributing a significant share of the worldwide market based on end-users. Moreover, it is to grow further during the forecast period due to market-boosting factors like the availability of portable POC devices, government initiatives, differentiation of healthcare services in different regions, and a rise in the number of CLIA-approved tests to diagnose various disorders.
The home healthcare segment is contributing a significant share of the market. It is likely to increase during the forecast period due to the emergence of economical and mobile POC testing devices and increasing demand for POC in home settings. In addition, it offers a high level of comfort to the patients.
REGIONAL ANALYSIS
The North American region is the leader in accounting for the highest global POC testing market share. In 2020, the North American market was worth USD 8340 Million, and it will continue the growing trends in the years to come. Unhealthy lifestyles, a high number of chronic diseases, a rising number of FDA approvals, and government regulations related to healthcare development are the key drivers behind the growth of POC diagnostics in North America. Canada shares the largest area in this region due to increased advanced point-of-care diagnostics and people caring more about their health. In Canada, scientists at the National Microbiology Laboratory (NML) are working to support providing point-of-care (POC) diagnostic testing devices for all levels of people at an affordable price. These POC devices are used mostly for tuberculosis, chlamydia, HIV, meningitis, and gastrointestinal diseases in this area. In 2020, the Pan-Canadian COVID-19 testing and screening guide different types of testing technologies using point-of-care diagnosis. Health Canada also gets approval from the U.S. Food and Drug Administration (FDA) for point-of-care testing and self-testing kits.
The market in Asia-Pacific is anticipated to grow at a CAGR of 10.2% during the forecast period. In the Asia Pacific, the market is expanding due to the rising prevalence of strategic initiatives taken by international players to improve healthcare infrastructure and increase awareness in developing countries. In the Asia-Pacific region, the market is expanding due to the rising prevalence of strategic initiatives taken by international players to improve healthcare infrastructure. In India, the Point-of-care diagnostics device market is increasing due to the use of these devices in monitoring ECG, oxygen saturation, blood pressure, and temperature, and increasing advanced POC devices. Key players, such as Siemens, Roche, and Abbot, are increasing investment in manufacturing POC diagnostic devices for homes, clinics, and hospitals. These manufacturers are producing portable, easy-to-use, affordable devices for people's convenience. Indian scientists and entrepreneurs have made more effort to develop next-generation POC diagnostic devices based on the latest technologies, such as smartphones and cloud-based devices. Researchers are going on a cancer diagnosis which high people are suffering in India. Nano biosensors are an excellent material for highly sensitive point-of-care (POC) cancer diagnostics. In China, the point of care (POC) market has started growing during the coronavirus period due to the focus of the healthcare system on in vitro diagnostics (IVD) and imaging testing. China is one of the largest manufacturers of these products and exports them to various countries. Government hospitals also use diagnostics. In China, the point of care (POC) market has started growing during the coronavirus period due to the healthcare system's focus on in vitro diagnostic (IVD). China is one of the largest manufacturers of these products and exports them to various countries. Government hospitals also use diagnostics tests to reduce the spread of the virus. In China, screening campaigns on targeted diseases such as HPV, prenatally evident conditions, hepatitis, and infectious emerging diseases such as avian flu and drug-resistant tuberculosis use these rapid POC tests to increase the market demand in this region.
Europe has a share of 32% due to viruses, increased adoption of advanced Point of care diagnostic devices, and increased awareness about innovative and advanced Point of care diagnostic devices. In recent years in Europe, diagnosing infectious diseases has increased to provide treatment in a timely manner. By using this point-of-care testing, infectious diseases can be identified quickly and controlled in a short period. In Europe, this point-of-care testing is used mainly during the pandemic time. In Europe, most infectious diseases such as influenza and HIV undergo point-of-care testing rapidly to detect the infection in the person in less time. Due to increases in the number of migrants in these regions, malaria is common. Therefore, more Europeans traveling to and from these nations may have impacted POCT for malaria. European Commission proposed using POCT for COVID-19 testing for the primary screening purpose. POCT's techniques include faster diagnosis times, improved hospital flow, and higher testing rates.
KEY MARKET PLAYERS
Notable market participants in the Global Point of Care Diagnostics Market profiled Abbott Laboratories, Inc., Danaher Corporation, Roche Diagnostics Limited, BioMerieux, Siemens Healthcare, Beckman Coulter, Inc., Becton, Dickinson and Company, Johnson & Johnson, Alere Inc., and PTS Diagnostics. Key market players are investing more in R&D to develop innovative products and expand revenues through collaborations with other companies, research universities, and private research organizations.
Siemens Healthineers, one of the leading companies in the POC diagnostics market, presents its customizable products for in vitro diagnostic tests designed to improve workflow, efficiency, and clinical outcomes. The company offers the latest innovations in Central Laboratories' Atellica® solutions, blood gas testing during treatment, and molecular diagnostics. The RAPIDPoint® 500e Blood Gas System2 provides an improved user experience and sets a higher standard for healthcare simplicity, quality, and data security. For labs interested in expanding the reach of molecular diagnostics, the new Fast Track Diagnostics test menu from Siemens Healthineers provides a syndrome panel to improve patient care and workflow.
RECENT HAPPENINGS IN THIS MARKET
- In October 2023, QIAGEN, announced the gain of CE certification for IVD kit and automated testing platform, NeuMoDx. The major aim of the launch is to enhance the company revenue and position in the market.
- In March 2023, bioLytical Laboratories announced its receival of Health Canada authorization for its INSTI Multiplex HIV-1/2 Syphilis antibody testing for use in point-of-care settings.
- In February 2023, bioMerieux received approval from U.S. FDA for its Biofire Spotfire respiratory panel system. The aim of the product launch is to expand the POC testing product portfolio of the company.
- In January 2023, Cipla announced the launch of Cippoint, a point-of-care device which offers testing for non-communicable, infectious diseases and various other disease conditions.
- In 2022, An agreement was announced between Una Health and MedTech, Siemens Healthineers for the distribution of Atellica VTLi Patient-side Immunoassay Analyser in primary care in the U.K. Una Health is high in manufacturing innovative point-of-care diagnostics and the improvement of the patient in healthcare settings.
- The Cobas Pulse System, Roche's newest generation of connected point-of-care solutions for advanced blood glucose monitoring, was launched in 2022. This system helps the high performance and smartphone usability of blood glucose meters.
- In 2022, Siemens Healthineers manufactured a CLINITEST Rapid COVID-19 Antigen Self-Test, approved by U.S. Food and Drug Administration for access at home.
DETAILED SEGMENTATION OF THE GLOBAL POC DIAGNOSTICS MARKET INCLUDED IN THIS REPORT
This market research report on the global point-of-care diagnostics market (POC testing market) has been segmented and sub-segmented based on the product, end-user, and region.
By Product
- Infectious Diseases
- Cardiac Markers
- Oncology Markers
- OTC Diagnostic Tests
- Drugs Of Abuse
- Blood Gas Testing
- Fertility Testing
- Urinalysis
- Coagulation
- Hematology
- Glucose Monitoring
- Ambulatory Chemistry
- Decentralized Clinical Chemistry
By End-User
- Clinics
- Hospitals
- Home Healthcare
- Ambulatory Care Settings
- Laboratories
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- The Middle East and Africa
Frequently Asked Questions
Which factors contribute to the growth of point of care diagnostics in Europe?
Factors such as the increasing prevalence of chronic diseases and the demand for rapid diagnostics contribute to the growth of point-of-care diagnostics market in Europe.
What are the emerging trends in Point of Care diagnostics in the Middle East and Africa?
The Middle East and Africa are witnessing a trend towards decentralized healthcare, driving the adoption of Point of Care diagnostics for quicker and more accessible medical results.
What are the challenges faced by Point of Care diagnostics companies in South America?
Challenges include navigating complex regulatory landscapes and addressing affordability concerns, which impact market penetration in South America.
How is the Point of Care diagnostics market in the Asia-Pacific region adapting to the increasing demand for personalized medicine?
The market is witnessing a shift towards the development of Point of Care diagnostics tailored for personalized medicine, reflecting the growing demand for individualized healthcare in the Asia-Pacific region.
Related Reports
Access the study in MULTIPLE FORMATS
Purchase options starting from
$ 2500
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1 888 702 9696 (U.S Toll Free)
Write to us: sales@marketdataforecast.com
